Editor’s note: Eye care venture PlenOptika found that contrary to common assumptions, the reason more people in the world don’t wear glasses isn’t from lack of need or because glasses are too expensive. Here we offer a case study on the US and Spanish startup that was first published in Demand magazine in Spring 2017.
Demand: ASME Global Development Review is shuttering after six years of publishing in-depth coverage of the most interesting manifestations of design, engineering and social ventures in global development. The biannual magazine was a premier source of global development writing and a sister publication to Engineering for Change. As an homage to Demand and the work of the experts whose voices it disseminated, we are reprinting Demand’s articles on this site.
A lot can happen in two years, so the company’s co-founder Shivang Dave provides four updates since first publication of this article.
- Plenoptika officially launched the QuickSee in India in last February and in the United States last October
- The company raised a small round of seed funding to cover manufacturing costs, regulatory approval, and the product’s initial commercial launch
- Pilots have begun with partners in 15 countries, including Kenya, Gambia and Micronesia
- The hardware is undergoing clinical studies for additional features
Outside of advanced economies, eye glasses are uncommon. There is a reason for that. Hardware startup PlenOptika’s investigation into why confronted its engineers with unexpected questions and issues.
Unlike the casually dressed students milling around Berkeley, Calif. coffee shops on a rainy January afternoon, Shivang Dave, PlenOptika’s CEO and co-founder, wore a sports a blazer and tie. Dave was dressed to pitch a potential investor, then after that meeting, he would get on a red-eye to Boston, where PlenOptika has a perch at Harvard’s Innovation Launch Lab, an incubator space for startups. Even though Dave has a PhD in Bioengineering and Biomedical Engineering, with an impressive assortment of fellowships and awards on his CV, he and the rest of his team are still running a tight ship, and Tuesday night red-eyes make the most sense on a US$20,000 per-year salary. “I earned more money as a graduate student,” he says ruefully.
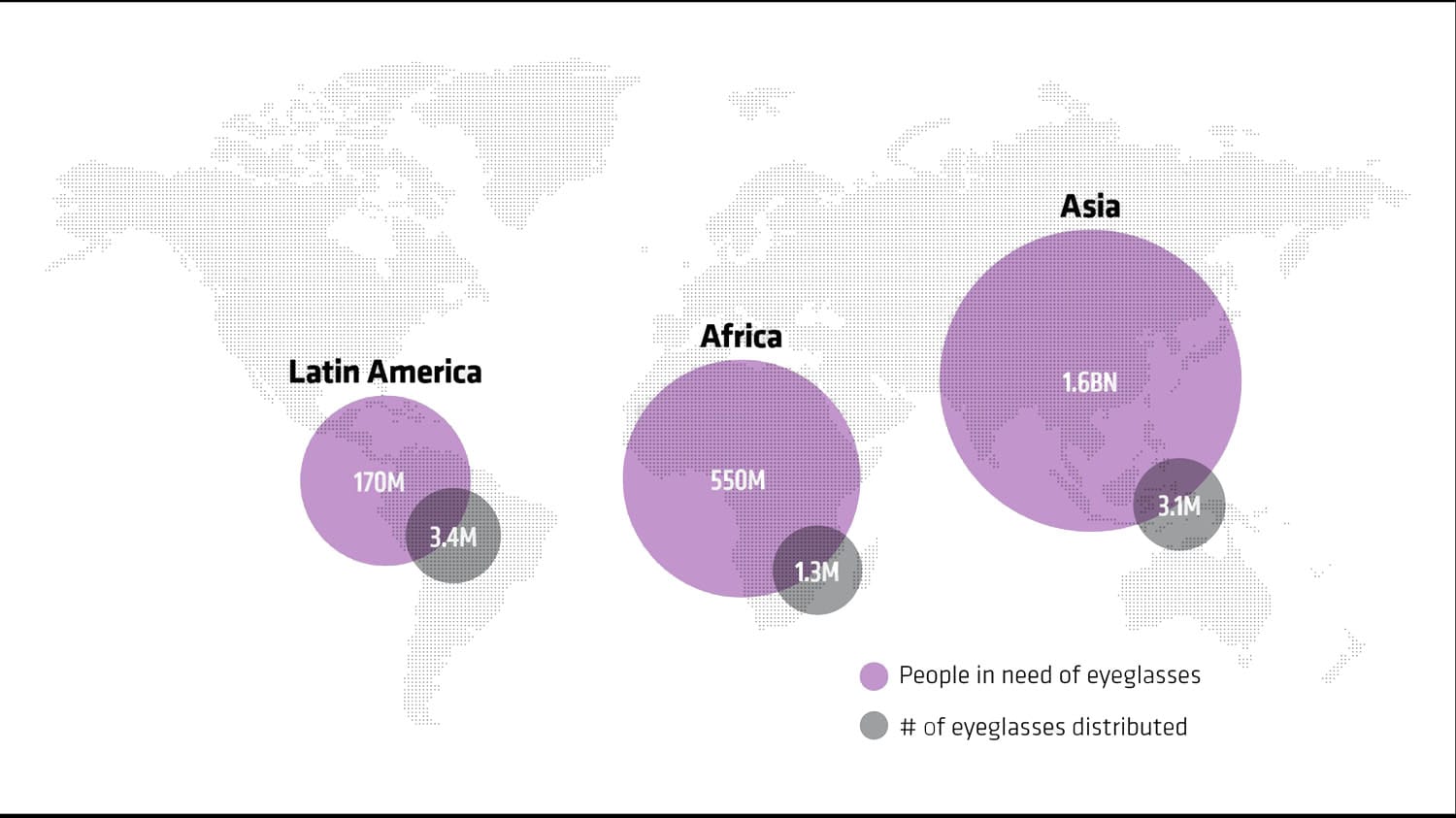
Eyeglasses need vs. distribution in Latin America, Africa and Asia
As a social entrepreneur, Dave’s primary objective with PlenOptika is not to strike it rich; it is to help “build innovative tools to provide eye care for all.” The idea for PlenOptika was born in 2011 with a mission of helping the 1.5 to 2.5 billion people around the world with less-than-perfect vision obtain glasses. (Of that figure, an estimated 153 million people suffer from such poor vision that they would be considered legally blind, according to the WHO.)
Dave began tackling this problem as a fellow with the Madrid-MIT M+Visión Consortium, a collaboration between the government of Madrid and MIT, whose purpose is to cultivate biomedical imaging and entrepreneurship around large-scale, unmet medical needs globally. The fellowship partnered Dave with Nicholas Durr and Daryl Lim, who had been working together on a technical solution to address the global glasses gap. Eduardo Lage, an electrical engineer and medical imaging specialist, also joined the team through the fellowship.
The solution the group devised is an eye examination device called the QuickSee, which looks a bit like a Viewfinder crossed with an oversized pair of binoculars. The QuickSee is a portable, low-cost, easy to use autorefractor— a well-established vision care technology that scans for abnormalities in the eyeball’s optical power. Anyone who has had an eye examination at a doctor’s office has likely had their eyes examined by an autorefractor. It is the objective part of a standard eye examination, and it works by shining light into the eye and then measuring how the light changes as it bounces off the back of the eye. The machine-based exam is usually followed by an eye care specialist testing a series of lenses and asking patients for feedback to fine tune their prescription. The QuickSee, by contrast, is designed to approximate a patient’s prescription as accurately as both the objective and subjective exams combined, minimizing the need for highly trained specialists in places where eye care is hard to access. What’s more, it is able to produce results quickly, in about 10 seconds per eye.
The investigative maze
When PlenOptika’s team started researching the global glasses shortage, their quest for information quickly evolved into a Matryoshka-like investigative maze, with every answer uncovering another question. Their starting point, was simply what prevented more people in the world from obtaining glasses if they needed them. Were they unaffordable to the global poor?
“Glasses are really expensive in the U.S., so the common belief was that cost must be the barrier to adoption [everywhere],” says Dave.
Indeed, about 25 years ago, glasses were universally expensive, he adds. But within the last 10 to 15 years, large global glasses manufacturers realized that billions of people living at the base of the global economic pyramid were an untapped market, and now, $2 and $3-per-pair glasses are widely available. (Glasses nevertheless remain expensive in advanced markets like the U.S. and Europe because of a near-monopoly on the market by one company, Dave explains.)
More than two billion people need glasses worldwide. 153 million of them could be considered legally blind.
If cost was not the issue, what was?
Statistics on trained eye care professionals around the world painted a compelling picture. Based on PlenOptika’s research, the number of optometrists per capita in the U.S. is 1:6,000; by contrast, in India, the ratio is 1:250,000 and in rural India, the ratio is 1:500,000. “There are [simply] not enough trained eye care professionals in many countries,” Dave says.
Why are there so few trained eye care professionals in places like India?
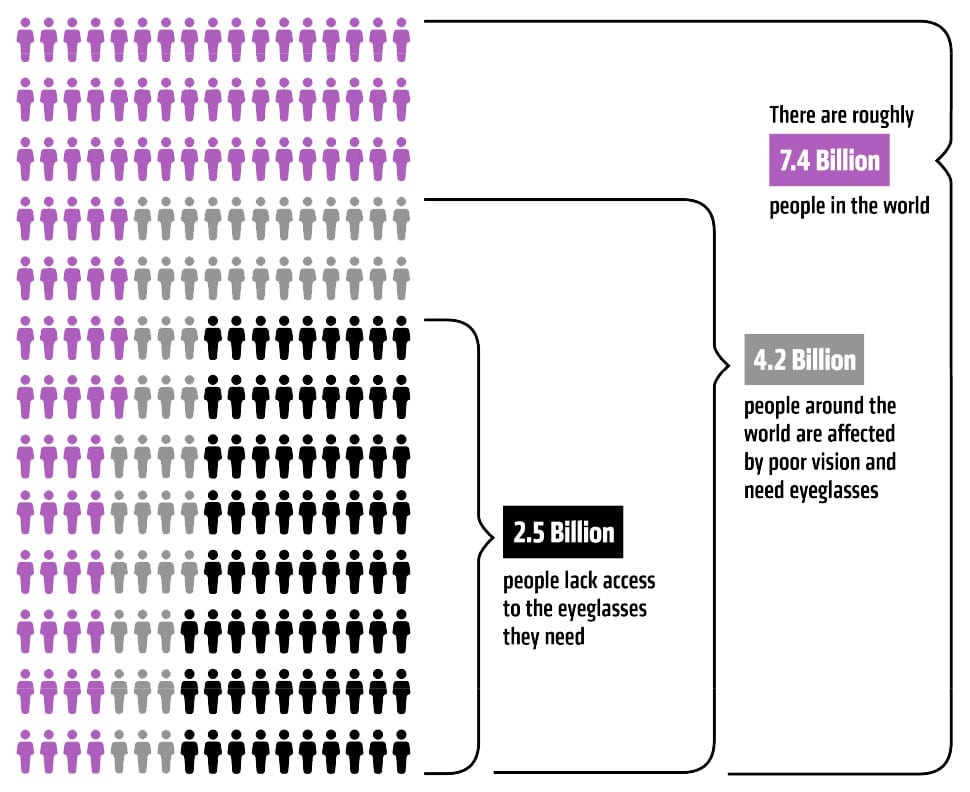
In the U.S., getting certified as an optometrist takes four years of schooling and can cost several hundred thousand dollars. That kind of time and cost would create market barriers anywhere. PlenOptika’s team wondered if professional training could be achieved more cheaply and quickly for markets like India where there is a shortage of experts.
The answer is yes, but not at the scale that is needed. Recruitment and training of qualified individuals takes six months to two years, in part because eye examination is not an entirely objective process. “There’s a bit of art to it, so even when someone is well-trained, it takes a few years of clinical practice to master the technique,” Dave explains.
Could technology replace or partly replace the need for trained eye care professionals?
Some aspects of routine eye examinations can be automated with the use of autorefractors. Autorefractors are staple machines in eye doctor offices in sophisticated care facilities worldwide. But they are expensive, costing around $10,000 each; they are also large, heavy pieces of equipment, and they are not always that accurate, Dave says. “They are used as a good starting point [in fine-tuning a prescription].” Hence the need for the subjective follow-up exam.
The PlenOptika team concluded that a “good starting point” for eye examinations may be sufficient to accelerate eye care access in underserved parts of the world, however. “We thought, if we can give healthcare workers an affordable and portable autorefractor, maybe these workers could be trained [more quickly] and go into rural areas to reach people,” Dave explains. Additionally, bringing down the cost of autorefractor technology might encourage existing optical centers in low-resource markets to invest in the machinery. (PlenOptika expects that the QuickSee will retail for less than half the $10,000 cost of standard autorefractors.)
Proving demand, not need
Often affordable product designers start from a different point than PlenOptika’s team did: they identify a need and then build a product they hope communities will adopt. The global development field is littered with products that seemed like game-changers, drew accolades and funding, and then languished on the shelf or in the field. The reason for failure typically comes down to lack of understanding of the market forces and barriers to adoption for a product. In other words, just because there is a need does not mean there is demand.
“A lot of people are making [frugal] medical devices right now, but they don’t really stop and think ‘will doctors adopt this?’” Dave says. “That’s a critical question, because a doctor might say that a signal you’re measuring is not clinically useful to me, or there are no reimbursements for it.”
The M+Visión program encouraged a different approach. Its goal was to build teams that could develop and take products to market in a relatively short time frame of three to four years. To meet this goal, it urged its fellows to fully flesh out the barriers of unmet medical needs as their first step in building a product and business plan. (PlenOptika was part of the inaugural M+Visión class. The program is now called MIT linQ and is run entirely by MIT.)
“[The program organizers] wanted us to spend a lot of time upfront understanding the clinical need and the real-life constraints that would influence the solution before starting to work on any technology,” says Durr, who is an assistant professor of biomedical engineering at Johns Hopkins University, in addition to working with PlenOptika. This approach allowed a measure of freedom and flexibility that most grant-based programs do not offer. “We got to form our own research program and act as the program’s principle investigators,” Durr says, rather than being gifted funds that were earmarked for a specific solution or goal.
The upfront investigative part of the project was nevertheless intense and punctuated with critical feedback from M+Vision’s expert advisors, whose job, Dave says, was “basically to attack” their idea. “We had to have as much evidence as we could that we would be successful in [translating our idea into a viable product] before we started the work,” adds Durr. That evidence included proving their technical and business model viability, demonstrating understating of clinical constraints, and mapping potential pitfalls down the line. “We [also] had to be able to justify to the consortium that what we were working on would be impactful if we were to succeed.”
For the first six months of the program, the fellows undertook exhaustive due diligence, which involved taking classes in biomedical imaging, interviewing global health experts, training and research with local hospitals in Madrid, and pitching ideas to an expert panel of faculty, clinicians, and industry experts every week. It took months of refinement to arrive at a proposal that was approved by the consortium. The team was then given a small grant to do a proof of principle for the technology, first identifying the potential clinical, research, adoption, business, and regulatory obstacles to getting to market. “The thinking was that only once we understood these [barriers] could we develop a solution that would address the
barriers effectively and move the needle on the [eye care] problem,” Dave says.
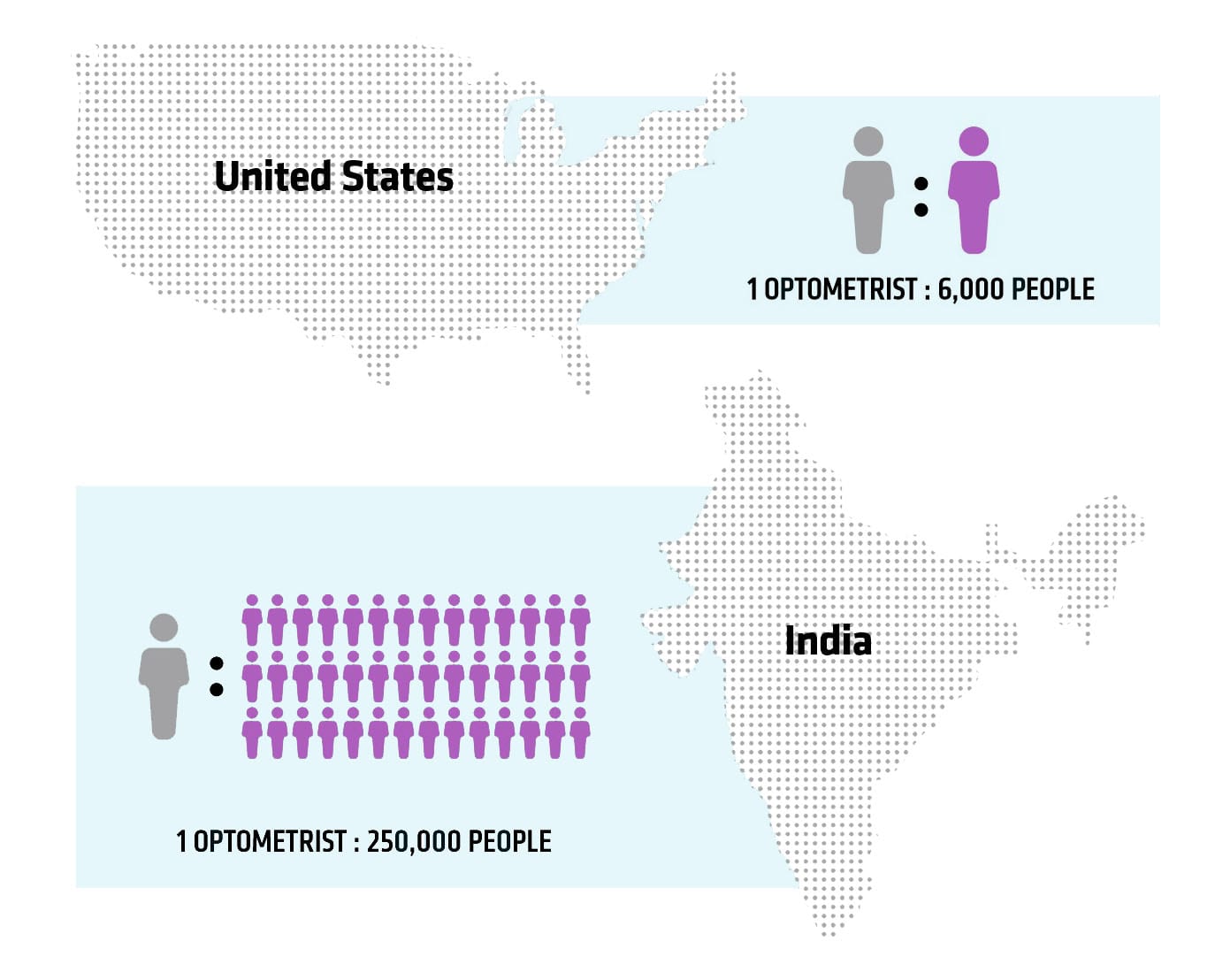
Availability of eye care specialists per capita
Low-cost portable eye care
The technology PlenOptika began testing—and ultimately used to build the QuickSee—relies on a method called “wavefront aberrometry,” which objectively measures the refractive errors in a patient’s eye, and which can diagnose optical problems. Like all wavefront aberrometers, the QuickSee works by shining a light into the eye, which generates a point of light on the retina at the back of the eye. The light then bounces off the retina, and the shape of the light as it exits the eye relays information about aberrations, or distortions, that are present. Some of these aberrations may indicate myopia or nearsightedness, hyperopia or farsightedness, or astigmatism, in which light is focused unevenly on the retina. Some conditions, like age-related presbyopia—a hardening of the lens that begins to affect most people in their 40s and 50s—can be corrected with generic off-the-rack glasses; the other disorders require tailored prescriptions.
To identify a patient’s prescription, a special camera called a “wavefront sensor” measures the shape of the light that comes out of the eye. Unlike a conventional camera that takes a single image, a wavefront sensor photographs the eye in segments and analyzes aberrations within each region of the eye. A computer in the device then processes all of the images collectively and calculates any refractive errors that might be present. With that, the QuickSee is able to approximate a prescription in about 10 seconds per eye, which it displays on-screen. The device can also transmit the results via Bluetooth to a smartphone or printer.
“We wanted to provide as many options as possible for as many scenarios as possible,” explains Durr. “In some places, the optometrist is involved with making the glasses. In others, they are just responsible for the prescription, and [the patient] takes the prescription to an optician who makes the lenses [for them].”
Each team member was responsible for a different aspect of the QuickSee’s design. Durr and Lim oversaw the optical design, which involved selecting the optical components to give the device maximum range and accuracy, then choosing a configuration that would optimally estimate a prescription while minimizing cost and device sensitivity. Lage focused on the electrical system and software. Dave’s role, meanwhile, encompassed the broader global health and entrepreneurial aspects of the project. The entire team collaborated on the mechanical aspects of the product.
Through field testing, the PlenOptika team found that the QuickSee produced results comparable to most two-part, objectivesubjective patient exams. This minimizes the need for low-resource eye care centers to stock both an autorefractor and phoropter— the lens device used in the patient feedback part of the exam. Dave is quick to note the QuickSee is for vision-testing only, however, and cannot replace a thorough eye exam that screens for diseases, like glaucoma or macular degeneration. But one of the device’s biggest benefits is that non-healthcare professionals can learn to use it in under an hour. The PlenOptika team hopes that this ease of use will be what enables more people to have vision problems diagnosed, particularly in areas where eye care professionals are in short supply.
PlenOptika began approaching stakeholders and potential customers only once they had a prototype to show and demonstrate. The rationale was that a theoretical product and a physical product will solicit different kinds of user input. “Early on, a lot of people will tell you that something is important, but they won’t give you serious or informed feedback until they get an actual working prototype,” Dave says. “Sometimes heavy customer testing isn’t needed in the initial ideation phase.”
There are exceptions. Affordable products like clean cookstoves and other non-medical devices are often culturally and regionally nuanced, which necessitates earlier user involvement. Also, customers for most affordable devices are sensitive to price, so it is important to understand the pricing parameters that can make or break a product in the market. That can be hard to do, however, without clarity on manufacturing costs, which most young companies do not have at the prototype phase.
“Field-testing is really useful, but I don’t think you can get a complete answer from it,” says Dave. “There are a lot of realities that you cannot answer at [the prototype] stage about final design, manufacturing costs, distributorships, import and exports fees—all of the in-the-weeds details that determine if you can sell a product at a sustainable price point.”
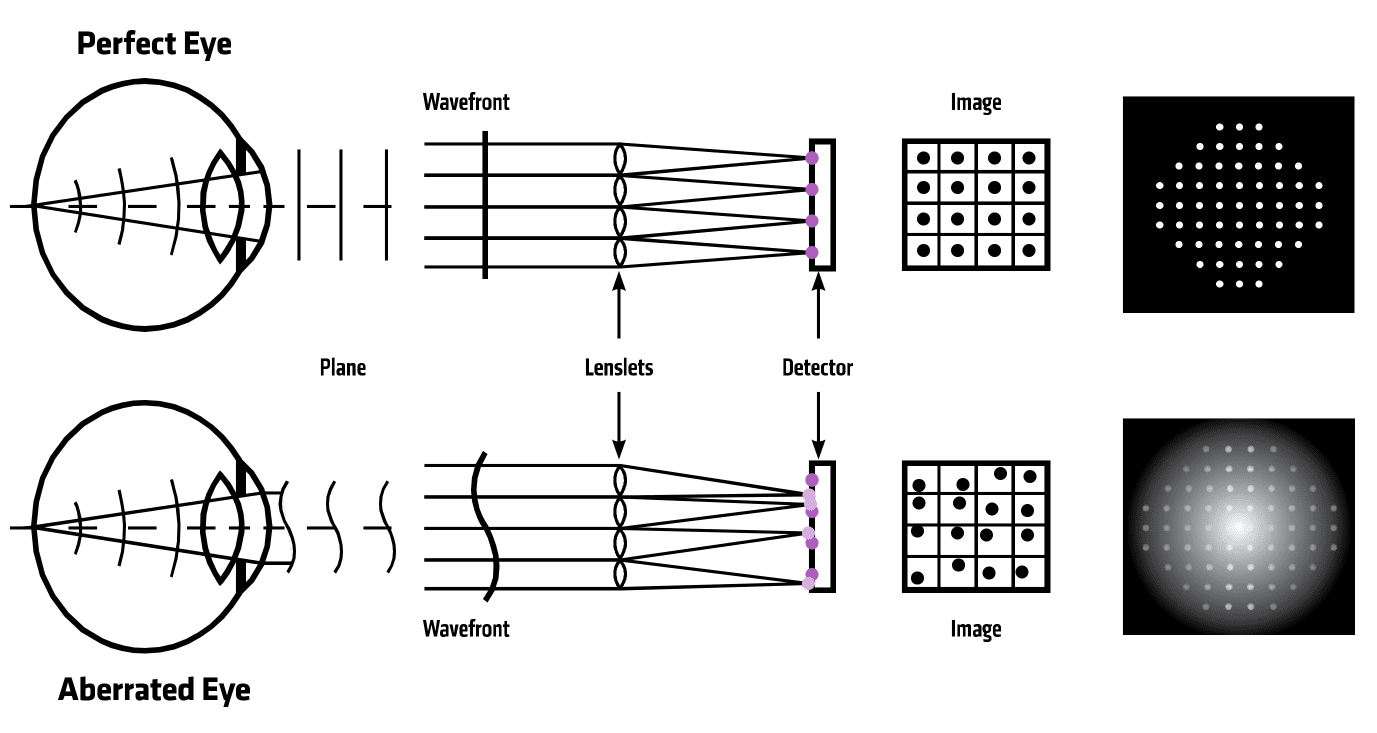
Autorefractor technology (wavefront aberrometry) used in PlenOptika’s QuickSee device
The market hurdle
In 2015, PlenOptika conducted a 708-patient clinical trial in partnership with specialist eye care provider Aravind Eye Hospital in the state of Tamil Nadu in India. The trial was funded by a grant from the United States-India Science & Technology Endowment Fund, a joint government initiative that largely focuses on healthcare technologies. The success of the clinical trial allowed PlenOptika to spin out of MIT and take strides towards manufacturing.
Potential users will tell you something is important, but they won’t give you serious or informed feedback until they get a working prototype.
It was also instrumental in exposing user experience issues that PlenOptika could not readily anticipate without putting the product in potential patients’ hands. For example, average nose size varies from one population to another, and that affected how the early QuickSee models fit against users’ faces. The team reengineered the eyecup with numerous molds and materials before “freezing” their design on one that worked universally.
There were also what Dave calls “anthropological issues,” as in India, where it is not customary for a young person to touch an elder person’s head (that is what elders do to young people to impart a blessing), or in some parts of the Middle East, where it is improper for a man to touch a woman’s head.
These are important issues to uncover when designing a medical device, even non-invasive ones, which have the lowest regulatory hurdles. PlenOptika is working toward U.S. FDA registration for the QuickSee. “Students don’t realize that when doing medical device design, you have to keep a lot notes and design history files. When you make a change, you have to justify that change” to clear regulatory scrutiny, Dave explains.
It is also critical to uncover design discoveries before manufacturing begins, given how costly manufacturing is for an early stage business. PlenOptika went through seven device iterations before freezing the design for manufacturing.
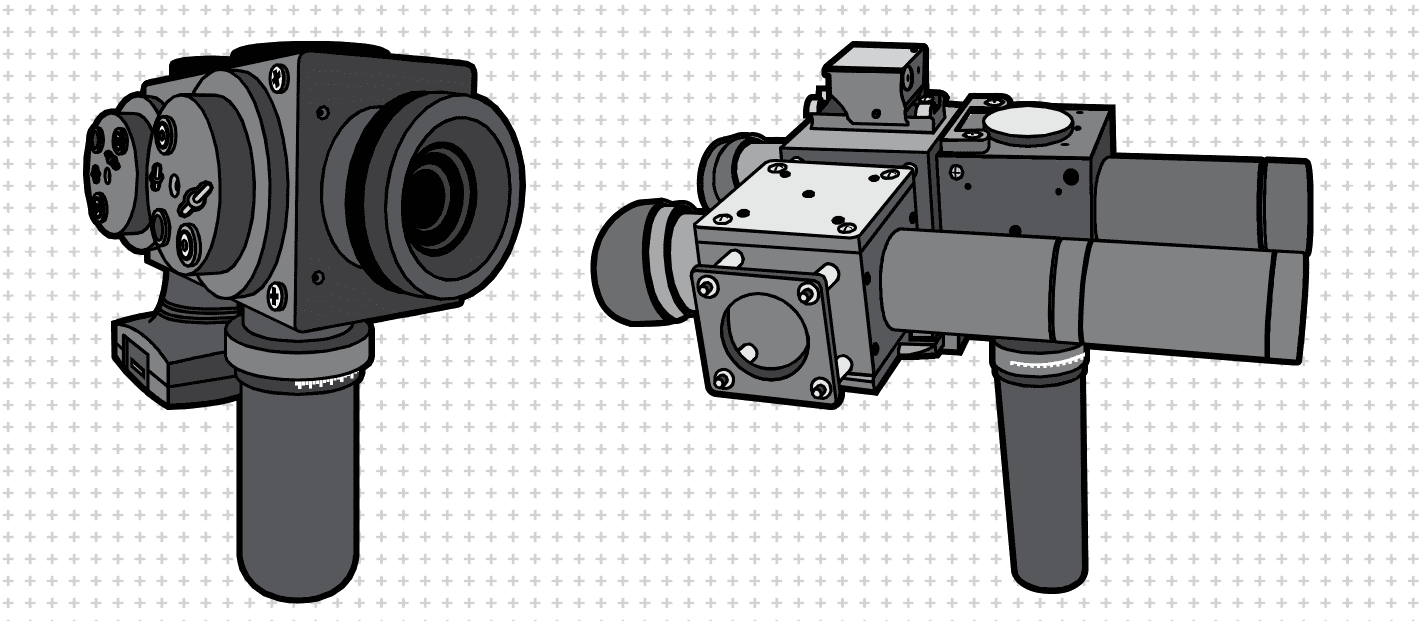
Early QuickSee prototypes
They have partnered with a reputable low-cost ophthalmic manufacturer called Aurolab, which Dave says gives their product credibility when pitching to customers and investors alike.
That, of course, is the big test for PlenOptika now, after having gone through the exhaustive exercise of pinpointing a problem, identifying a market, and developing and testing a technically-viable solution. PlenOptika’s target customers are eye care professionals who can use the QuickSee as another tool in their eye care toolbox. In this respect, partners like Aravind Eye Hospital, which has been working to expand rural healthcare delivery through mobile clinics, rural centers, and telemedicine, make sense for PlenOptika. The team is also targeting healthcare organizations that might not have an eye care professional on staff.
PlenOptika has gotten this far with just under $2 million in grant funding over the past five and a half years. In that time, a number of other low-cost vision care products have emerged, both hardware-based and app-based. Most envision “disrupting” the eye care space, rather than helping eye care specialists expand their services and reach, as PlenOptika hopes to do. “We are trying to work with eye care professionals to augment their care,” Dave says. “That’s the training we received academically, and that’s what we believe. That’s the brand we have built. The partners we have reflect that.”
Dave adds that the app-based products in particular have complicated PlenOptika’s pitch to investors. Investing in a software firm may be attractive for investors from a risk standpoint, because their development and overhead costs are lower, and they can get to market sooner than hardware startups. But the eye tests tend to be less accurate, Dave explains, because most rely on a prior prescription to use as a baseline for approximating a new prescription. For a global market, that kind of solution cannot serve people who have never before had an eye exam.
In addition, advanced markets like the U.S. and Europe have strict healthcare regulations, and currently the only way to obtain anything other than reading glasses is through a prescription issued by a licensed practitioner. This necessitates optometrist buy-in for products that are trying to impact today’s healthcare environment, regardless of who and where those products’ target customers are. “I think working with eye care specialists gives us a more stable foundation to be successful,” Dave says, while acknowledging that this is not the billion-dollar strategy that investors typically prefer.
Now, selling that message is the part of the maze PlenOptika has to navigate.
About the Author
Adrienne Day is a writer, editor and New York native. She has written for The New York Times, New York magazine, the Village Voice, Stanford Social Innovation Review, and Wired, among other outlets, and has worked as an editor at EW and Spin magazines. Adrienne holds a bachelor’s from SUNY Binghamton and a master’s in journalism from Columbia University.

God awarded us with our senses to whom we utilize to explore the world. Object sensation caused by our first glance. All of us have a sense of vision that we utilize to explore the beauty of nature. But most people lose this sensation by some fatal diseases like diabetes. Diabetes disease occurs with an increase in blood sugar. This disease leads to blindness when it affects eyes.
In the early era, People loss their sense of vision due to the effect of diabetic disease and It was impossible to diagnose the diabetic eye and disease progression leads to irreversible blindness.
SEE THE COMPLETE ARTICLE FROM THIS LINK
Diagnose Of The Diabetic Eye Using Artificial Intelligence