Agriculture
January 18, 2024
Waterbackpack PAUL
Read SolutionImplemented by
Prof. Franz-Bernd Frechen, University of Kassel
Updated on February 14, 2024
·Created on August 23, 2016
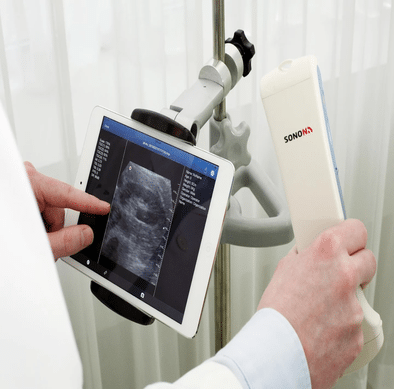
The Healcerion SONON 300C Portable Ultrasound is an ultrasound device that utilizes iOS and Android technology to view and transmit ultrasound images and recordings.
The Healcerion SONON 300C Portable Ultrasound is an ultrasound device that utilizes iOS and Android technology to view and transmit ultrasound images and recordings. The device is intended for diagnostic ultrasound echo imaging, measurement, and analysis of the human body for clinical applications including fetal/OB, gynecology, and general (abdominal) imaging. Information for this product was provided courtesy of the WHO’s 2016 Call for Innovative Health Technologies for Low-Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$6,000.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
Healcerion
Competitive Landscape
Direct competitors include GE LOGIQ V2 and GE VScan Pocket Ultrasound.
Regions
Worldwide
Manufacturing/Building Method
Mass production.
Intellectural Property Type
Patent
User Provision Model
Customers can fill out the online contact form the Healcerion website to request a quote. The device can also be obtained from distributors in Europe, North America, South East Asia, and the Middle East. The Sonon 300C product is typically sold to hospitals, private doctors, NGOs, and students.Interview with representative
Distributions to Date Status
More than 400 units have been distributed across over 30 countries as of August 2016.Interview with representative
Power supply type
Rechargeable Li-ion battery (2600 mAh)
Consumables
Ultrasound gel
Indispensable equipment for function (Y/N)
iOS or Android mobile device
Maintenance or calibration required by user at time of use? (Y/N)
No
Scanning modes
2D
Clinical application
Fetal/OB, Gynecology, Abdominal
Display
iOS or Android mobile device
Probe frequency (MHz)
3.5
Design Specifications
The SONON 300C uses a paired tablet or smartphone with the Healcerion mobile application to display ultrasound images. Images are wirelessly transmitted to the tablet or smartphone from where they can be analyzed and shared with others. The SONON 300C allows user to transmit images and recordings to the paired tablet or smartphone via Wi-Fi, 3G, or LTE networks. The mobile application can be downloaded for free from the Apple App Store or the Google Play Store. All collected images and data are stored in the mobile app and cannot be recovered if the app is removed and reinstalled.
The software enables ultrasound image capture and review, controls for time gain, dynamic range, display of mirror image, focal length, depth, brightness, contrast, linear/elliptical measurement, and image annotation, as well as storage and email transmission of images and videos. The SONON Ultrasound Imaging System allows the user to image in real time and review cine or freeze-frame images in a 2-dimensional scan format. The ultrasound imaging system also utilizes Pulsed Wave Doppler for determining the depth and location of tissue interfaces.
The Sonon 300C uses a 3.5 MHz convex array transducer that supports a max depth of 20cm and a field of view of 58.2°. The Sonon 300C has an operating time of 2.1 hours in scan mode and 12 hours in stand-by mode. The battery takes 3 hours to completely recharge. Accessories for the device (battery, back-up battery, adapter, battery charger, and power cord) are included with device.
Dimensions: 78mm x 219mm x 38mm
Weight: 390g (with battery)
Operating temperature/humidity: 0-35°C / 30% to 75% RH
Mobile device operating system and software requirements: iOS 7.0 or later, Android 4.01 or later
Minimum mobile device requirements: iPhone 5, iPad 3rd generation or later version, Galaxy S3, Galaxy Note 2, Galaxy Note 10.1 generation or later version
Technical Support
Only authorized personnel shall perform any type of repair on the Sonon 300C. While it is better for users to be trained on how to interpret ultrasound images, this knowledge is not required to operate the Sonon 300C because images can be sent via Wi-Fi or mobile data for expert interpretation. It takes approximately 1-2 hours to train an individual on how to use the Sonon 300C device and mobile application. A warranty of 1-3 years is provided with the product. The duration of warranty varies depending on the situation.Interview with representative
Replacement Components
Replacement parts are currently not available.Interview with representative
Lifecycle
The device has an anticipated lifetime of 2-5 years.
Manufacturer Specified Performance Parameters
Unknown.
Vetted Performance Status
Unknown.
Safety
Do not use the Sonon 300C on a patient while the device is charging. Additional safety precautions are listed in the Healcerion web.
Complementary Technical Systems
Unknown.
Academic Research and References
None
Won, Ryu and Chan, Choung, “MOBILE ULTRASOUND DIAGNOSIS PROBE APPARATUS FOR USING TWO-DIMENSION ARRAY DATA“, EP2841936 (A1) ? 2015-03-04, European Patent Office, 2015.
Healcerion, “Sonon 300c App“, Google Play, 2017.
Healcerion, “Indications of Use“, Food and Drug Administration, DEPARTMENT OF HEALTH AND HUMAN SERVICES, 2015.
Compliance with regulations
CE marked, FDA, KFDA (Korean Food and Drug Administration).
Evaluation methods
Non-clinical tests showed compliance with standards for electrical safety, electromagnetic compatibility, RF wireless capabilities, acoustic output levels, clinical measurement range and accuracies, display performance, usability, failure mode and risk analyses, biocompatibility, and software evaluation and cybersecurity management.
Other Information
Designers should also investigate additional guidelines, such as the WHO Manual of diagnostic ultrasound. Designers should also take social factors into consideration, such as the use of ultrasounds for gender-biased sex selection in some parts of the world.

Agriculture
January 18, 2024
Implemented by
Prof. Franz-Bernd Frechen, University of Kassel

Agriculture
November 30, 2024
Implemented by
KOKO Networks

Agriculture
January 30, 2024
Implemented by
IslaUrbana

Agriculture
June 24, 2024
Implemented by
Tiny Totos

Agriculture
January 27, 2024
Implemented by
Palinter
Agriculture
February 2, 2024
Implemented by
PHILOS

Agriculture
February 20, 2024
Implemented by
SurgiBox

Agriculture
June 29, 2024
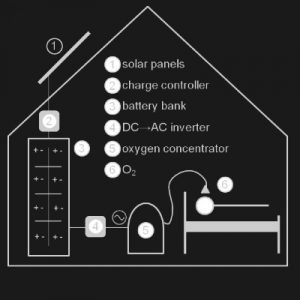
Agriculture
February 27, 2024
Implemented by
Solar Oxygen

Agriculture
May 20, 2024
Have thoughts on how we can improve?
Give Us Feedback