
Agriculture
February 29, 2024
Oxygen Reservoir System
Read SolutionImplemented by
Diamedica

Updated on March 8, 2024
·Created on August 31, 2021
Mobile oxygen generators for use in emergency medical clinics. Courtesy of WHO Compendium 2021
PCI Deployable Oxygen Supply Systems are commercial-grade oxygen suppliers for hospitals and clinics. This product is available in three capacities: 80 L/min, 200 L/min, and 500 L/min. The product includes remote monitoring of the control system via 3G or Wi-Fi connection.
This product was selected for inclusion in WHO’s 2021 Compendium of Innovative Health Technologies for Low‐Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$163,800.00
Target Users (Target Impact Group)
Distributors / Implementing Organizations
None
Competitive Landscape
Direct competitors include Solar-Powered Oxygen Delivery (SPO2) and O2 Cube.
Regions
Worldwide
Manufacturing/Building Method
PCI manufactures its products in production facilities in California, USA; Curitiba, Brazil; and Bangalore, India.
Intellectural Property Type
Patent
User Provision Model
Direct sales to hospitals and medical centers
Distributions to Date Status
As of 2020, this product has been installed in over 100 medical facilities worldwide.
Design Specifications
This produce uses Vacuum Swing Adsorption (VSA oxygen) technology to collect, compress, and supply oxygen to a medical clinic. This product is designed to be "plug and play" and function automatically. Connection is made into the hospital oxygen pipeline to major users (e.g., ICU, ER, OP) in permanent or temporary situations. Medical oxygen is then supplied on-demand with flow adjustment from 25 - 100% of system capacity.
Technical Support
Provided by the manufacturer
Replacement Components
Provided by the manufacturer
Lifecycle
15-20 years
Manufacturer Specified Performance Parameters
The manufacture designed this product to be automatic, portable, and durable for emergency settings.
Vetted Performance Status
This product has been vetted by the manufacturer to comply with the following performance standards:
Safety
Unknown
Complementary Technical Systems
The system connects to clinical equipment that requires an oxygen supply.
Academic Research and References
None
Pacific Consolidated Industries, “On-Site Oxygen Solutions“
Compliance with regulations
The device complies with ISO 7396-1:2016, ISO 9001 & 13485, EU Medical devices directive Class IIb Council Directive 93/42 EEC, 006/42 EC Machinery Directive, 97/23/EEC Pressure Equipment Device (PED), and 2006/95/EC Low Voltage Electrical Equipment Directive (LVD).
Evaluation methods
Unknown
Other Information
None

Agriculture
February 29, 2024
Implemented by
Diamedica
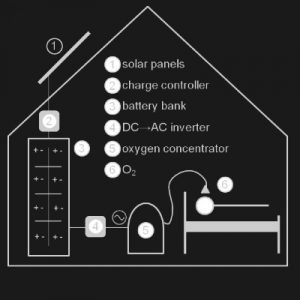
Agriculture
February 27, 2024
Implemented by
Solar Oxygen

Agriculture
February 5, 2024
Implemented by
Gradian Health Systems
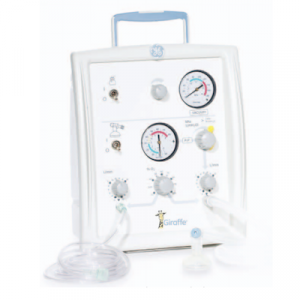
Agriculture
November 22, 2023
Implemented by
GE (General Electric)
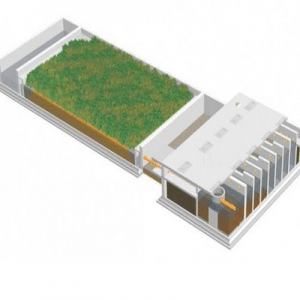
Agriculture
January 11, 2024
Implemented by
BORDA

Agriculture
January 16, 2024
Implemented by
George Greene III, PE, PhD

Agriculture
December 18, 2023
Implemented by
Clivus Multrum

Agriculture
March 7, 2024

Agriculture
January 10, 2024
Implemented by
Niwa

Agriculture
January 25, 2024
Implemented by
RoadPower Inc
Have thoughts on how we can improve?
Give Us Feedback