
Agriculture
December 2, 2024
EcoZoom Jet
Read SolutionImplemented by
BioLite
Updated on December 19, 2023
·Created on August 27, 2015
DSJI ensures primary immunization from infections using needle-free injections for developing countries.
The PATH disposable-syringe jet injector (DSJI) is a sterile needle-free device that uses a single-dose syringe and a pressurized liquid stream rather than a needle to penetrate through the skin and deliver injections to the intradermal, subcutaneous, or intramuscular tissues. The PATH DSJI aims to reduce needle reuse and the associated disease spread due to shortages of sterile needles and trained lab workers. An estimated 23.5 million new HIV, hepatitis B, and hepatitis C infections occur every year through needle reuse and accidental needle stick injuries.

Agriculture
December 2, 2024
Implemented by
BioLite

Agriculture
September 27, 2024
Implemented by
Birthing Kit Foundation
Agriculture
December 19, 2023
Implemented by
Hadleigh Health Technologies and Rice University
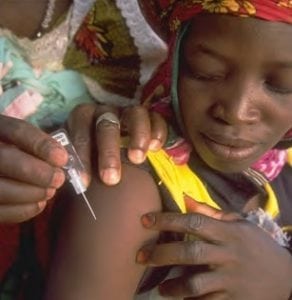
Agriculture
December 7, 2023
Implemented by
PATH

Agriculture
September 27, 2024
Implemented by
PATH

Agriculture
June 23, 2024
Implemented by
LoooP Creative Ltd

Agriculture
January 10, 2024
Implemented by
NRSRelief

Agriculture
December 31, 2023
Implemented by
Potential Energy

Agriculture
June 22, 2024
Implemented by
World Bicycle Relief

Agriculture
June 8, 2024
Implemented by
ClickMedix
Have thoughts on how we can improve?
Give Us Feedback