
Agriculture
November 22, 2024
AEST Agricultural Waste Charcoal Briquettes
Read SolutionImplemented by
Appropriate Energy Saving Technologies Limited (AEST)

Updated on March 8, 2024
·Created on August 31, 2021
A portable X-ray machine that screens for pulmonary health issues. Courtesy of WHO Compendium 2021
The Reveal 35C portable dual energy X-ray detector screens and aids in the diagnosis of pulmonary health issues including tuberculosis, lung cancer, COPD, pneumonia, and COVID-19. It is used in a similar manner to conventional digital x-ray detectors.
This product was selected for inclusion in WHO’s 2021 Compendium of Innovative Health Technologies for Low‐Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$124,999.99
Target Users (Target Impact Group)
Public Sector Agencies
Distributors / Implementing Organizations
KA Imaging
Competitive Landscape
Direct competitors include GlobalDiagnostiX.
Regions
Worldwide
Manufacturing/Building Method
Unknown
Intellectural Property Type
Patent
User Provision Model
Direct from manufacturer
Distributions to Date Status
Unknown
Clinical application
X-rays
Indispensable equipment for function (Yes/No)
No
Consumables
No
Device weight (kg)
3.2 kg
Device dimensions
459.5 x 383.5 x 15 mm
Max power consumption (W)
63 W
Power supply type
Lithium battery
Maintenance or calibration required by user at time of use? (Yes/No)
Yes
Type of technology used for test
X-ray
Time required for procedure
Unknown
Design Specifications
Reveal 35C uses a three layer stacked sensor design to produce three X-ray images using a single conventional chest X-ray exposure. The X-ray images can then be used to generate a conventional digital radiography, a bone subtracted and a soft tissue subtracted X-ray image, and lateral view dual energy X-ray images.
The device is intended to be used in a similar way as a conventional digital X-ray detector.
Technical Support
Provided by the manufacturer and trained general technicians
Replacement Components
Lithium battery
Lifecycle
5-10 years
Manufacturer Specified Performance Parameters
Enable bone and soft tissue images in a single X-ray exposure
Vetted Performance Status
There is an ongoing lung cancer clinical trial with 30 patients at Grand River Hospital and a second, IRB-approved COVID-19 clinical trial with 600 patients with Princess Margaret Hospital and University of Toronto that began in June 2020. The product has since been FDA 510(k) cleared and Health Canada approved and is available for sale in the USA and Canada. Reveal 35C has also been certified under several performance standards.
Safety
Standard precaution with X-ray devices of safety factors such as radiation exposure should be taken
Complementary Technical Systems
A laptop or computer is needed to operate the device, an anti scatter grid can be supplied upon request
Academic Research and References
Fredenberg, E., 2018, “Spectral and dual-energy X-ray imaging for medical applications.” Nuclear Instruments and Methods in Physics Research, Vol. 878, pp. 74-87.
Maurino, S. L., Ghanbarzadeh, S., Ghaffari, S., and Karim, K. S., 2019, “Novel multi-energy x-ray detector allows for simultaneous single-shot acquisition of digital radiography and tissue-subtracted images.” European Congress of Radiology-ECR.
Wong, H. Y. F., et al., 2020, “Frequency and distribution of chest radiographic findings in patients positive for COVID-19.” Radiology, Vol. 296(2), pp. 72-78.
National Library of Medicine, “Feasibility of Multi-Energy Digital Radiography Detector for Lung Lesions Detection“, Nacional Center for Biotechnology Information, 2020
Compliance with regulations
EC 60601-1:2005, COR1:2006, COR2:2007, AMD1:2012 Medical electrical equipment — Part 1
IEC 60601-1-2:2014 (Ed 4.0) Medical electrical equipment — Part 1-2
ISO 14971:2007 Medical Devices - Application Of Risk Management To Medical Devices
IEC 62220-1-1 Edition 1.0 Medical Electrical Equipment-Characteristics Of Digital X-Ray Imaging Devices Part 1-1
Evaluation methods
Clinical Trials
Other Information
None

Agriculture
November 22, 2024
Implemented by
Appropriate Energy Saving Technologies Limited (AEST)

Agriculture
February 5, 2024
Implemented by
Centre for Vision in the Developing World

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 1, 2024
Implemented by
Infantrust Parenting Solutions

Agriculture
February 16, 2024
Implemented by
Equalize Health
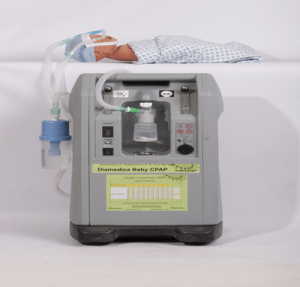
Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
December 2, 2024
Implemented by
BioLite

Agriculture
December 3, 2024
Implemented by
Veryday (McKinsey Design)

Agriculture
February 8, 2024
Implemented by
GE Healthcare

Agriculture
January 8, 2024
Implemented by
Healing Waters International
Have thoughts on how we can improve?
Give Us Feedback