
Agriculture
November 22, 2024
AEST Agricultural Waste Charcoal Briquettes
Read SolutionImplemented by
Appropriate Energy Saving Technologies Limited (AEST)

Updated on September 27, 2024
·Created on September 26, 2019
Infaclip, previously known as SafeSnip, is an obstetric device that simultaneously cuts and clamps the umbilical cord while reducing exposure to unintended bloodborne pathogens in areas where facilities are scarce.
Infaclip, developed by NOvate Medical Technologies (acquired by BTG International), previously known as SafeSnip, is a low-cost device intended to prevent infection-related neonatal deaths in developing countries. The designers claim that it can be used in regions where home births are common, and infants are susceptible to infections caused by unsanitary birth conditions. It is a three-inch disposable plastic clamp that cuts, seals, and disinfects an umbilical cord in one step. The second step in the operation of the device is the separation of the two halves. After the cord is clipped, SafeSnip breaks in two, leaving one half of the device clamped onto the baby’s umbilical cord to seal the wound while the other is discarded.
Target Regions
Africa, Southeast Asia
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Community, Small and Medium-sized Enterprises
Distributors / Implementing Organizations
This product has been developed and implemented by NOvate Medical Technologies, LLC, (acquired by BTG International), which is a New Orleans-based medical device development company focused on commercializing high-quality, low-cost medical products.
Competitive Landscape
Direct competitors include PATH Safe Delivery Kit.
Regions
Africa, Southeast Asia
Manufacturing/Building Method
SafeSnip is produced by Novate Medical Company and is in the development stage in Galway, Ireland.
Intellectural Property Type
Select Type
User Provision Model
The product is distributed by Novate Medical Company, which is a development stage medical device company in Galway, Ireland.
Distributions to Date Status
None yet, in partnership with John Snow, Inc. (JSI), Novate is requesting validation funding to develop a comprehensive training program and to evaluate the usability and effectiveness of SafeSnip in Nepal.
Guide type provided
Yes
Shelf life (years)
Unknown
Six 'cleans' covered (Y/N)
Yes
Surgical components
Surgical clip only
Units per package
One
Design Specifications
SafeSnip is an obstetric device used to cut and clamp the umbilical cord simultaneously in the birthing process. The device is made from plastic and is safe in application to prevent neonatal mortality due to umbilical cord infections while reducing unintended bloodborne pathogen exposure. The design also makes it possible for the device to be used only once. Available solutions in the market do not have sterilized blades or clips provided in the medical kits, leading to infections. The device is mass produced using plastic polymer for retail under 1 USD. After the application, SafeSnip breaks in two, leaving one half of the device firmly clamped onto the baby's umbilical cord to seal the wound while the other is discarded.
Technical Support
Provided by the manufacturer
Replacement Components
The device is for single time use and does not have any replaceable components
Lifecycle
The device is for single-time use
Manufacturer Specified Performance Parameters
Designer specified performance targets include: safe, reliable, single time use, and low-cost.
Vetted Performance Status
Unknown
Safety
Product should not be reused to avoid contamination
Complementary Technical Systems
Product should be used by reliable healthcare professionals and midwives
Academic Research and References
Collins, J.H. and Woodhead, R.G., 1989, U.S. Patent No. 4,856,517, Washington, DC: U.S. Patent and Trademark Office.
Dickinson, S. J., 1966, U.S. Patent No. 3,247,852, Washington, DC: U.S. Patent and Trademark Office.
Kinmond, S., Aitchison, T.C., Holland, B.M., Jones, J.G., Turner, T.L., and Wardrop, C.A., 1993, Umbilical cord clamping and preterm infants: a randomised trial, Bmj, 306(6871), pp. 172-175.
Compliance with regulations
Unknown
Evaluation methods
The product is in the development phase and third-party field trials are currently being performed.
Other Information
None

Agriculture
November 22, 2024
Implemented by
Appropriate Energy Saving Technologies Limited (AEST)

Agriculture
February 5, 2024
Implemented by
Centre for Vision in the Developing World

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 1, 2024
Implemented by
Infantrust Parenting Solutions

Agriculture
February 16, 2024
Implemented by
Equalize Health
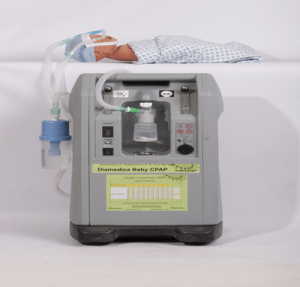
Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
December 2, 2024
Implemented by
BioLite

Agriculture
December 3, 2024
Implemented by
Veryday (McKinsey Design)

Agriculture
February 8, 2024
Implemented by
GE Healthcare

Agriculture
January 8, 2024
Implemented by
Healing Waters International
Have thoughts on how we can improve?
Give Us Feedback