
Agriculture
November 22, 2024
AEST Agricultural Waste Charcoal Briquettes
Read SolutionImplemented by
Appropriate Energy Saving Technologies Limited (AEST)
Updated on March 12, 2024
·Created on November 8, 2022
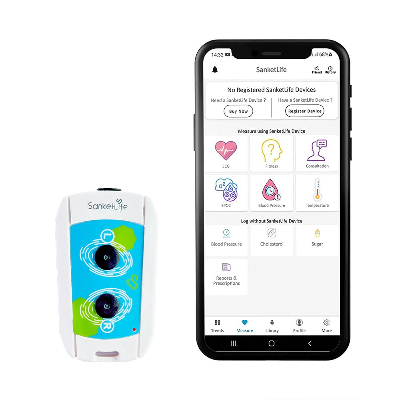
A portable wireless device for ECG and multi-parameter capture.
SanketLife is a clinically grade, touch based 12-Lead ECG monitor which can be carried anywhere and can be operated by anyone with minimal or no training. Since it is capable of taking full 12-Lead ECG, it is apt for use by doctors, healthcare professionals, diagnostic services and users and patients equally. Along with ECG, this portable Heart monitor can measure accurate heart rate, heart rate variability, stress and more than a hundred heart diseases.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$125.00
Market Suggested Retail Price (Secondary Currency)
49.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
This product is distributed by Agatsa Software Pvt. Ltd.
Competitive Landscape
Direct competitors include Spandan ECG and Cardio-Pad.
Regions
Worldwide
Manufacturing/Building Method
SanketLife is mass-produced in India.
Intellectural Property Type
Patent
User Provision Model
This product can be bought directly from manufacturer.
Distributions to Date Status
Unknown
Signal measurement range (mV)
+/- 300 mV
Frequency response (Hz)
0.5 Hz to 100 Hz
Sampling rate (Hz)
Unknown.
Filters
Unknown.
Measurements
Unknown
Consumables
CR2 batteries
Internal storage (number of ECGs)
Storage is related to the smartphone used with the app
Power Supply Type
CR2 Battery
Indispensable equipment for function (Y/N)
No
Maintenance or calibration required by user at time of use? (Y/N)
No
Design Specifications
SanketLife is intended to be a portable wireless device, able to provide ECG reports. Aiming to that, it is light (~45 g) and small (96 x 50 x 22 mm), using Bluetooth connectivity to send the patterns taken through a 12 lead ECG to an Android or iOS app. In has In-App interpretations of the results, which are supported by AI-driven technology. It also works with a Li-Ion coin battery with a capacity of 600 mAh. Detailed specifications can be found the website.
Technical Support
Provided by the manufacturer.
Replacement Components
Provided by the manufacturer.
Lifecycle
2-year free-replacement warranty.
Manufacturer Specified Performance Parameters
Diagnostic accuracy variables such as sensitivity, specificity, negative and positive predictive value, negative and positive likelihood ratios needed to be estimated.
Vetted Performance Status
A prospective diagnostic test accuracy trial was conducted in outpatient settings of a tertiary cardiac care centre in India. A total of 100 patients, attended cardiology OPD, were included in the study. Consecutive ECGs were taken by 12 lead standard ECG as well as by SanketLife ECG.
Safety
Since Sanketlife is a monitoring device, qualifies as a Class II medical device, with risks associated with its precision to provide accurate measurements. This device complies with the ISO 13485:2016, and Agatsa is working with different agencies for US FDA clearances and CE validations because of the high demand for small ECG machines in the international markets.
Complementary Technical Systems
SanketLife Mobile App
Academic Research and References
Kumar, S., 2020, “Assessment of diagnostic accuracy of SanketLife e A wireless, pocket-sized ECG biosensor, in comparison to standard 12 lead ECG in the detection of cardiovascular diseases in a tertiary care setting“, Indian Pacing Electrophysiol J., 20(2), pp. 54–59.
Kumar, Ayushman, “Agatsa, world’s smallest ECG maker, eyes entry into US market next year“, Money Control, 2022
Compliance with regulations
ISO 13485:2016, USFDA 510(K) in process, ETSI EN 300 328 V1.91:2015, CE, EC13. IEC 61000-4-2:2008,IEC 61000-4-3:2007.
Evaluation methods
A prospective diagnostic accuracy trial was conducted in a tertiary care setting, a total of 100 patients underwent for both type of ECG (a standard 12 lead ECG as well as SanketLife) consecutively. The primary objective of the study was to ascertain the diagnostic accuracy and reliability of the
SanketLife ECG in comparison to standard 12 lead ECG. The patients either came to the cardiac tertiary care centre with symptoms suggestive of cardiovascular disease or have a history of it. All patients who were referred for 12 lead standard ECG, also got their consecutive ECG done with SanketLife. ECG variables namely Heart Rate, PR, QRSD, QT & QTc were monitored, displayed and saved in a cloud system and compared against certain heart abnormalities visual against traditional 12 LEAD ECG machine.
The analysis showed a high degree of agreement and accuracy of SanketLife in detecting major cardiovascular conditions (Major Minnesota codes) such as Left and right bundle branch block, ST-segment elevation and ST-segment depression, AV conduction block. SanketLife showed high
sensitivity (98.15%) and specificity (100%) in diagnosing major cardiovascular conditions.
Other Information

Agriculture
November 22, 2024
Implemented by
Appropriate Energy Saving Technologies Limited (AEST)

Agriculture
February 5, 2024
Implemented by
Centre for Vision in the Developing World

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 1, 2024
Implemented by
Infantrust Parenting Solutions

Agriculture
February 16, 2024
Implemented by
Equalize Health
Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
December 2, 2024
Implemented by
BioLite

Agriculture
December 3, 2024
Implemented by
Veryday (McKinsey Design)

Agriculture
February 8, 2024
Implemented by
GE Healthcare

Agriculture
January 8, 2024
Implemented by
Healing Waters International
Have thoughts on how we can improve?
Give Us Feedback