
Agriculture
June 23, 2024
AguaPallet
Read SolutionImplemented by
LoooP Creative Ltd

Updated on February 20, 2024
·Created on October 3, 2020
SurgiBox is an ultraportable sterile field that fits in a backpack.
SurgiBox is a surgical technique that combines the benefits of state-of-the-art operating rooms with comprehensive personal protective equipment, in one ultraportable package. SurgiBox’s platform technology integrates seamlessly into surgical workflows to make surgery safer for patients and providers alike.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$132.00
Target Users (Target Impact Group)
Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Unknown
Countries
United States
Manufacturing/Building Method
This product is currently in the prototyping phase and not yet manufactured at scale.
Intellectural Property Type
Patent
User Provision Model
The designers have not yet selected their user provision model.
Distributions to Date Status
None
Equipment Provided
High visibility plastic, arm port, adhesive drape, surgical tray, fan, and filter
Method of Sterilization
Fan and filter prevents external infection
Shelf Life
5-10 years
Number of Successful Surgeries
None
Type of Surgery
Urgent surgery without medical device
Design Specifications
Features
Technical Support
Provided by the manufacturer
Replacement Components
Replaceable components include the battery for the fan.
Lifecycle
5 - 10 years
Manufacturer Specified Performance Parameters
Designer specified performance targets include: user-friendly, lightweight, durable, portable, and low-cost.
Vetted Performance Status
No testing has been completed
Safety
Users must take appropriate precautions when using a fan.
Complementary Technical Systems
None
Academic Research and References
Elisha, M., Shafagh, M., et al., 2014, “Intraoperative fluoroscopy, portable X-ray, and CT: patient and operating room personnel radiation exposure in spinal surgery.” The Spine Journal, 14, pp. 2292-2294.
Mark, L., 2014, “Commentary on: Intraoperative fluoroscopy, portable X-ray, and CT: patient and operating room personnel radiation exposure in spinal surgery.” The Spine Journal, 14, pp. 2985-2991.
Mulconrey, D., 2016, “Fluoroscopic Radiation Exposure in Spinal Surgery.” Clinical Spine Surgery, 29, pp. 331-335.
Create the Future, “SurgiBox: The Operating Room in a Backpack“, 2018
Compliance with regulations
The product is planning to get a certificate by US FDA General Controls and similar regulations.
Evaluation methods
Unknown
Other Information
None

Agriculture
June 23, 2024
Implemented by
LoooP Creative Ltd

Agriculture
January 10, 2024
Implemented by
NRSRelief

Agriculture
December 31, 2023
Implemented by
Potential Energy

Agriculture
June 22, 2024
Implemented by
World Bicycle Relief

Agriculture
June 8, 2024
Implemented by
ClickMedix
Agriculture
February 14, 2024
Implemented by
USTAR Biotechnologies (Hangzhou) LTD

Agriculture
January 8, 2024
Implemented by
Gadgil Lab, University of California Berkeley

Agriculture
December 3, 2024
Implemented by
Envirofit International
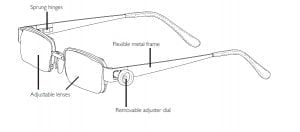
Agriculture
February 5, 2024
Implemented by
EyeJusters

Agriculture
February 8, 2024
Implemented by
GE Healthcare
Have thoughts on how we can improve?
Give Us Feedback