
Agriculture
February 6, 2024
UC Berkeley CellScope
Read SolutionImplemented by
Center for Information Technology Research in the Interest of Society and the Banatao Institute
 discontinued
discontinued
Updated on December 11, 2023
·Created on June 13, 2017
This product has been discontinued.
This product has been discontinued.
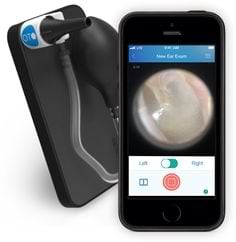
The CellScope Oto device is a smartphone otoscope system that includes an attachment to convert a standard smartphone into a digital otoscope with data transmission capabilities. Requires CellScope Oto Mobile App to record & share images and/or videos of the ear canal.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$299.00
Target Users (Target Impact Group)
Community, Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
Clinicians and families may purchase the Oto directly from the Cellscope website.
Countries
United States
Manufacturing/Building Method
Oto is mass produced by CellScope.
Intellectural Property Type
Patent
User Provision Model
Users can obtain the Oto from the product website. There is also an adapter for a standard insufflator bulb (not included).
Distributions to Date Status
Unknown
Design Specifications
The Oto is an optical attachment that transforms an iPhone into a digital otoscope. The device includes an insufflator port for pneumatic otoscope and uses LEDs.
The device is primarily intended for use in children as ear infections are most prevalent in children under 7 years of age, although reusable tips come in custom sizes for all ages
Accessories include:
Technical Support
CellScope provides FAQ, video vutorials, and instructions on their website, as well as contact information.
Replacement Components
Compatible with standard Welch-Allyn specula
Lifecycle
While a traditional otoscope can last several years, with changing iPhone sizes and phones often being upgraded or changed yearly, the lifecycle of an Oto may be much shorter.
Manufacturer Specified Performance Parameters
Provide diagnostic solutions at the same rate as any digitally enhanced microscope in a well-equipped hospital
Vetted Performance Status
Physicians can diagnose otitis media equally well with either modality (traditional otoscope or the oto), and thought the images from the oto were more than adequate for diagnosing otitis media
Safety
Safety instructions are provided with purchase.
Complementary Technical Systems
The Seymour Application for viewing the inside of the ear and communication with doctors.
Academic Research and References
Douglas Kamerow, Regulating medical apps: which ones and how much?, BMJ 2013, 347
Kathryn M. Rappaport, C. M. McCracken and J. Beniflah, Assessment of a Smartphone Otoscope Device for the Diagnosis and Management of Otitis Media, Clinical Prediatrics 2016 Voll 55(9) 800-810
JR Patrick, How mHealth will spur consumer-led healthcare, MHealth Journal, July 2015
Lynne Shallcross, Mobile Health: Apps for every age and ouch, May 2015
Kate Stephenson, Claude Laurent, OPEN ACCESS GUIDE TO AUDIOLOGY AND HEARING AIDS FOR OTOLARYNGOLOGISTS
Compliance with regulations
Oto is HIPPA compliant. The Oto’s status as a Class 1 device exempts it from FDA approval or clearance (Source: 21 CFR Parts 862-892) due to the low-risk level associated with this type of device. CellScope adheres to the FDA’s rules and requirements for marketing, including but not limited to: registration, labeling and good manufacturing practices.
Evaluation methods
Manufacturer cites satisfaction among doctors and families as evaluation criteria.
Other Information
In order to talk back and forth with doctor, an at-home user must have a network connection (WiFi or cell data)

Agriculture
February 6, 2024
Implemented by
Center for Information Technology Research in the Interest of Society and the Banatao Institute

Agriculture
September 27, 2024
Implemented by
MamaOpe

Agriculture
December 4, 2023
Implemented by
Cure Tunisia

Agriculture
June 7, 2024
Implemented by
Bahmni Coalition

Agriculture
January 8, 2024
Implemented by
YakuPura

Agriculture
September 27, 2024
Implemented by
Biospectal

Agriculture
January 2, 2024
Implemented by
Uzima

Agriculture
February 21, 2024
Implemented by
Flexmo

Agriculture
June 6, 2024
Implemented by
Hello Doctor

Agriculture
December 19, 2024
Implemented by
Green Heat
Have thoughts on how we can improve?
Give Us Feedback