Agriculture
June 23, 2024
AguaPallet
Read SolutionImplemented by
LoooP Creative Ltd
Updated on February 23, 2024
·Created on August 21, 2020
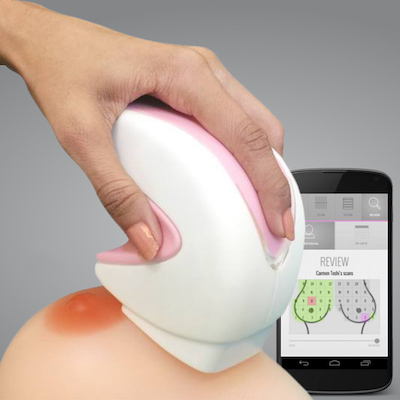
iBreastExam is a rapid diagnostic for early-stage detection of breast abnormalities.
iBreastExam is a low cost, battery-powered, handheld rapid diagnostic device used for the identification of breast abnormalities and early detection of breast cancer. The device is radiation-free and is non-invasive. The product is being commercially distributed in over 25 countries across Africa and South Asia.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$10,000.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
GE Healthcare is the primary distributor. iBreastExam has partnered with 40+ private clinics, governmental institutions, non-profits, and corporate social responsibility initiatives to enable the use of the product and breast examinations.
Competitive Landscape
Direct competitors include BreastIT.
Regions
Africa, South Asia
Countries
Botswana, Mexico, Nepal, Oman
Manufacturing/Building Method
The product is mass-manufactured in India.
Intellectural Property Type
Patent
User Provision Model
As of now, the product has been used through large-scale governmental initiatives as partnerships between the manufacturer and governmental, national, or international institutions. Users can request a demo through the manufacturer's website.
Distributions to Date Status
As of July 2020, 200+ products have been installed in health care settings and 300,000+ women have been screened.
Consumables
N
Detection sensitivity
83-86%
Indispensable equipment for function (Y/N)
N
Maintenance or calibration required by user at time of use? (Y/N)
N
Number of Tests Performed
Over 1,000
Power supply type: Continuous, Recharging only (V, time required, battery life), Other
Rechargeable battery
Time required for procedure (minutes)
<10 minutes
Design Specifications
The iBreastExam is a handheld device for breast abnormality detection. The device uses a ceramic sensor technology that is able to detect subtle variations in tissue elasticity, which corresponds to the detection of lumps in the breast tissue. The device is battery operated, wireless, and radiation-free. A typical scan takes about 5 minutes and the data from the device is immediately sent to a smartphone, where user data is stored. The device goes over the four quadrants of the breast during an exam and colours are used to communicate possible abnormalities to the operator. Green represents normal tissue and red indicates a possible abnormality and need for further assessment. The colours are shown both by an LED on the device itself as well as on a breast map on the smartphone. The device can detect tissue abnormalities as small as 3-5 mm.
It takes 4-8 hours to train a health care worker on the operation of the device, after which the worker takes a test before being certified.
Technical Support
Provided by the manufacturer. Regional distributors also provide product servicing.
Replacement Components
There are no replaceable components.
Lifecycle
2-5 years
Manufacturer Specified Performance Parameters
Manufacturer specified performance targets include portability, low cost, scalable, standardized, accessible, and easy to use.
Vetted Performance Status
There have been three clinical studies using the iBreastExam which showed the following approximate averages: 86% sensitivity, 90% specificity, 63% PPV, 89% NPV.
Safety
Device safety training is included in the general device training provided to health care workers.
Complementary Technical Systems
A smartphone is required for data transmission and saving. A reliable power source is required to recharge the battery in the device.
Academic Research and References
S. P. Somashekhar, R. Vijay, R. Ananthasivan, and G. Prasanna, Noninvasive and Low-Cost Technique for Early Detection of Clinically Relevant Breast Lesions Using a Handheld Point-of-Care Medical Device (iBreastExam): Prospective Three-Arm Triple-Blinded Comparative Study, Indian J. Gynecol. Oncol., vol. 14, no. 2, pp. 1–6, Jun. 2016, doi: 10.1007/s40944-016-0057-1.
R. B. Broach, R. Geha, B. S. Englander, L. DeLaCruz, H. Thrash, and A. D. Brooks, A cost-effective handheld breast scanner for use in low-resource environments: A validation study, World J. Surg. Oncol., vol. 14, no. 1, p. 277, Oct. 2016, doi: 10.1186/s12957-016-1022-2.
Cousins, Sophie, “A New Way to Detect Breast Cancer“, New York Times, 2018
Tyagi, Chhavi, “Backed by GE Healthcare, this startup’s handheld device is a revolutionary cancer detection tech“, The Economic Times, 2017
UE LifeSciences, “iBreastExam Brochure“
Compliance with regulations
The iBreastExam has CE and Unicert ISO13485 certification and has been cleared by the FDA.
Evaluation methods
The device has been evaluated through three clinical trials and has been evaluated against current standard breast examination techniques including mammogram, clinical breast exam, and ultrasound.
Other Information
The iBreastExam has won several awards: 2019 Lyfebulb-Helsinn Oncology Innovation Award, 2019 Best Pitch Innov8 Talks Arab Health Award, 2018 Bayer Foundation Grants 4 Impact Award.

Agriculture
June 23, 2024
Implemented by
LoooP Creative Ltd

Agriculture
January 10, 2024
Implemented by
NRSRelief

Agriculture
December 31, 2023
Implemented by
Potential Energy

Agriculture
June 22, 2024
Implemented by
World Bicycle Relief

Agriculture
June 8, 2024
Implemented by
ClickMedix
Agriculture
February 14, 2024
Implemented by
USTAR Biotechnologies (Hangzhou) LTD

Agriculture
January 8, 2024
Implemented by
Gadgil Lab, University of California Berkeley

Agriculture
December 3, 2024
Implemented by
Envirofit International
Agriculture
February 5, 2024
Implemented by
GE Healthcare

Agriculture
June 22, 2024
Implemented by
Hippo Roller
Have thoughts on how we can improve?
Give Us Feedback