
Agriculture
September 27, 2024
Diamedica Helix Portable Ventilator
Read SolutionImplemented by
Diamedica

Updated on March 8, 2024
·Created on August 31, 2021
A mechanical ventilator designed in response to the COVID-19 pandemic.
The MVM Ventilator is a mechanical invasive ventilator that was designed for mass production in response to the COVID-19 pandemic. The ventilator is nearing the end of its prototyping and testing phase as of 2020 and the manufacturer has plans to deliver 10,000 ventilators to Canada in 2021.
This product was selected for inclusion in WHO’s 2021 Compendium of Innovative Health Technologies for Low‐Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$6,000.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
As of 2020, Vexos and the Government of Canada
Competitive Landscape
Direct competitors include Impala Ventilator and CERN HEV Ventilator.
Regions
Worldwide
Manufacturing/Building Method
MVM Ventilators are manufactured by Vexos Inc., an international electronics manufacturing company that has facilities in the United States, Canada, China, and Vietnam.
Intellectural Property Type
Select Type
User Provision Model
Direct sales to governments and hospitals
Distributions to Date Status
None as of 2020, but a contract has been signed to provide 10,000 units to the Canadian government.
Use case (category and target population)
Intensive Care; adult (> 40 kg)
Ventilation modes
PCV, PSV
Internal PEEP capability and range
No (External Disposable PEEP Valve)
Range pressure setting
2-50 cmH2O
Peak pressure limitation
Yes
FiO2 settings
Adjustable, External, Measured
Inspiratory/expiratory pause maneuver
Yes
Primary display modes
Peak pressure, tidal volume, ventilator mode status
Spirometry available
Yes
Capnography available
No
Main alarms available
Yes
Air source, O2 low pressure capability
External, No
Internal or external battery (operating time, recharging time)
Internal (2 hours, 24 hours)
Power requirements (W)
60 W
Design Specifications
The MVM ventilator is inspired by the Manley ventilator, which was developed by Roger Manley in 1961. The ventilator includes the following main components: Connection to oxygen and air supply, sintered filters, gas blender, four differential pressure sensors, air/oxygen delivery proportional valve, adjustable pressure limiting valve, negative pressure relief valve, oxygen sensor, spirometer, braking system (connected to the tracheal tube), condensate trap, silicone membrane filter, expiration valve, and PEEP valve.
The control system regulates the air/oxygen delivery proportional valve and the expiration valve based on the selected programmed respiratory cycle.
The following accessories are needed: Gas blender (GENTEC GMX120 or equivalent) and catheter mount (DEAS 1510TG, DEAS 22xxDV, or equivalent).
The control system regulates the air/oxygen delivery proportional valve and the expiration valve based on the selected programmed respiratory cycle.
Technical Support
Support is available through the manufacturer.
Replacement Components
Replacement components are available from the manufacturer.
Lifecycle
5-10 years
Manufacturer Specified Performance Parameters
The MVM ventilator was designed in response to the COVID-19 pandemic and thus the designers focused on the following targets: (1) Small number of components, (2) ease of procurement, (3) simplicity of construction, (4) low cost, (5) convenience of deployment, (6) customizability, (7) reliability, and (8) limited oxygen consumption.
Vetted Performance Status
The ventilator has passed all of the ISO 60601 tests for basic safety and essential performance.
Safety
For safety, the manufacturer specifies the following operational ranges: Temperatures from 10 to 40 degrees Celsius, and in relative humidities from 10-95%, at a maximum altitude of 3000 meters.
Complementary Technical Systems
The manufacturer offers additional accessories, including a gas blender for mixing medical air and oxygen before the inlet to the mechanical ventilator.
Academic Research and References
Compliance with regulations
The device was designed under the MHRA Rapidly Manufactured Ventilator System guidelines and has received approval from Health Canada as well as initial approval from the U.S. FDA, as of 2020.
The mechanical ventilator conforms to ISO 60601-1, 60601-1-2, 60601-1-6, 60601-1-8, ISO 80601-2-80, ISO 10993, ISO 18562-1,2,3.
The manufacturer plans to certify the software according to standard EN 62304.
Evaluation methods
Performance testing was carried out as specified by the ISO 80601-2-12:2020 Testing Protocol and a usability check has been performed according to standard EN 62366.
Other Information
None

Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
February 2, 2024
Implemented by
Gradian Health Systems
Agriculture
March 8, 2024
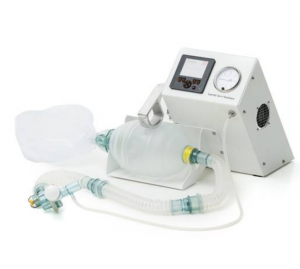
Implemented by
Laerdal Global Health

Agriculture
March 8, 2024
Implemented by
MTTS

Agriculture
March 8, 2024
Implemented by
Conseil Européen pour la Recherche Nucléaire (CERN)

Agriculture
February 29, 2024
Implemented by
Motivation

Agriculture
December 29, 2023
Implemented by
Sawyer
Agriculture
November 24, 2023
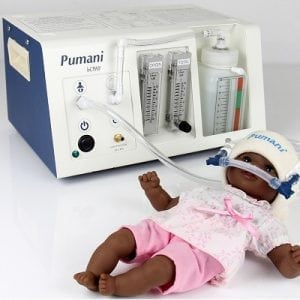
Implemented by
Hadleigh Health Technologies, and Rice 360

Agriculture
December 19, 2023
Implemented by
RealRelief
Agriculture
June 26, 2024
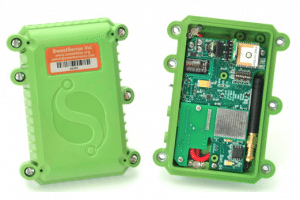
Implemented by
SweetSense Inc
Have thoughts on how we can improve?
Give Us Feedback