
Agriculture
September 27, 2024
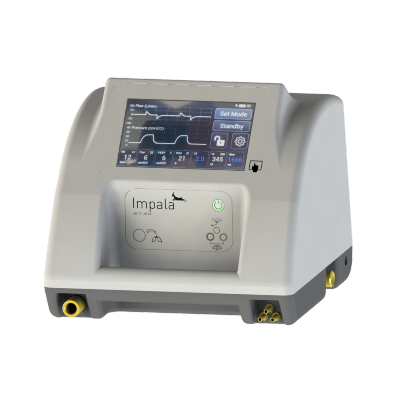
Diamedica Helix Portable Ventilator
Read SolutionImplemented by
Diamedica
Updated on March 8, 2024
·Created on August 31, 2021
Ventilator designed for interhospital, intrahospital, or prehospital emergency transport.
The Impala Ventilator supports respiratory functions and is designed for use in interhospital, intrahospital, or prehospital emergency transport contexts.
These units consist of a flexible breathing circuit, a control system, monitors, and alarms. Power can be supplied via internal batteries, an external battery, or electricity.
This product was selected for inclusion in WHO’s 2021 Compendium of Innovative Health Technologies for Low‐Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$450.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
MTTS-Asia
Competitive Landscape
Direct competitors include Laerdal Servi Ventilator and MVM Ventilator.
Regions
Worldwide
Manufacturing/Building Method
Unknown
Intellectural Property Type
Trade Secret
User Provision Model
The product is under clinical investigation as of June 2020, a user provision model has not been selected yet
Distributions to Date Status
None
Use case (category and target population)
Intensive Care; adult, child (> 5 kg)
Ventilation modes
PCV, VCV, CPAP/BiPAP, SIMV, PSV, PRVC
Internal PEEP capability and range
Yes (0-25 cm H20)
Range pressure setting
5-60 cm H2O
Peak pressure limitation
Yes
FiO2 settings
Adjustable, Internal (21-100%), Not Measured
Inspiratory/expiratory pause maneuver
No
Primary display modes
PEEP, peak pressure, tidal volume, ventilator mode status
Spirometry available
Yes
Capnography available
No
Main alarms available
No (High/low MV not present)
Air source, O2 low pressure capability
Internal (Pump), No
Internal or external battery (operating time, recharging time)
Internal (3 hours, 1 hour)
Power requirements (W)
60 W
Design Specifications
To operate, users first check that the unit is ready for use by running performance and calibration checks. They then make sure that settings for all components, including alarms, are correct and appropriate for the patient type and condition.
The patient can then be connected to the ventilator, where caregivers are responsible for monitoring the patient during the ventilation.
Main components include the MTTS-Asia Impala Ventilator, a gas hose with an oxygen connector, a power cable, and a silicon mask.
Technical Support
A trained general technician can be contacted for technical support
Replacement Components
Nasal mask, batteries
Lifecycle
5-10 years
Manufacturer Specified Performance Parameters
Designed for use in emergency settings as well as a controlled hospital environment, precise and reliable device to provide safe gas flow delivery, long-life batteries.
Vetted Performance Status
Compliance with standards including 60601-1 and 60601-2
Safety
The ventilator should only be operated by a trained professional.
An alternative means of ventilation should be available in case of an malfunction with the primary ventilator.
Operating the device outside of the specified ambient temperature range
(19°C - 37°C) or humidity range (30%RH - 90%RH) can compromise
performance.
The ventilator should not be operated if any of the components appear to be damaged or broken. Damaged or broken components should be discarded and replaced.
For additional safety considerations, refer to the user manual provided by the manufacturer.
Complementary Technical Systems
A nasal mask is required
Academic Research and References
None
MTTS, “Impala Ventilator User Manual“, 2020
Compliance with regulations
Awaiting approval for CE mark
Evaluation methods
Quality testing
Other Information
None

Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
February 2, 2024
Implemented by
Gradian Health Systems
Agriculture
March 8, 2024
Implemented by
Laerdal Global Health

Agriculture
March 8, 2024
Implemented by
Mechanical Ventilator Milano

Agriculture
March 8, 2024
Implemented by
Conseil Européen pour la Recherche Nucléaire (CERN)

Agriculture
June 23, 2024
Implemented by
LoooP Creative Ltd

Agriculture
January 10, 2024
Implemented by
NRSRelief

Agriculture
December 31, 2023
Implemented by
Potential Energy

Agriculture
June 22, 2024
Implemented by
World Bicycle Relief

Agriculture
June 8, 2024
Implemented by
ClickMedix
Have thoughts on how we can improve?
Give Us Feedback