Agriculture
September 27, 2024
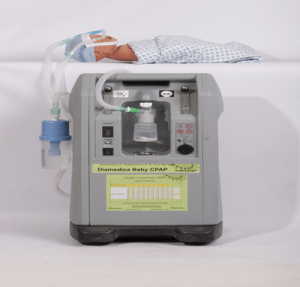
Diamedica Baby CPAP
Read SolutionImplemented by
Diamedica

Updated on September 27, 2024
·Created on September 7, 2016
The Diamedica Helix Portable Ventilator is a portable, gas-driven ventilator.
The Diamedica Helix Portable Ventilator is a pneumatically-operated ventilator that can be driven by an oxygen concentrator, reserve oxygen cylinder, or air compressor. The device is available in two versions: adult/pediatric and pediatric/neonate.
Target Regions
Africa, Central Asia, East Asia, North Asia, South Asia, Southeast Asia
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$4,900.00
Target Users (Target Impact Group)
Community, Small and Medium-sized Enterprises
Distributors / Implementing Organizations
Diamedica
Regions
Africa, Central Asia, East Asia, North Asia, South Asia, Southeast Asia
Manufacturing/Building Method
The product is designed and manufactured in the UK.
Intellectural Property Type
Select Type
User Provision Model
Users can obtain this product directly from the manufacturer.
Distributions to Date Status
Unknown
Consumables
N
Power supply type
A/C, vehicle D/C, battery backup
Indispensable equipment for function
Y
Maintenance or calibration required by user at time of use? (Y/N)
Y
Display Type
Analog
Gas Flow Range (L/min)
2-48
Pressure Range (cm H2O)
8-40
Patient population (specify)
Adult, paediatric
Design Specifications
The ventilator is a gas-driven, electrically-powered ventilator. It is a time-cycled, volume limited, pressure ventilator with vertically mounted bellows inside a polycarbonate case and driven by a pneumatic piston. It can be used for both short- and long-term ventilation.
Technical Support
Provided by the manufacturer
Replacement Components
The components that make patient contact are made from autoclavable materials and as such can be reused. The main replacement components include a self-inflating bag, silicone tube, and a 22F- 15M straight connector.
Lifecycle
Unknown
Manufacturer Specified Performance Parameters
Manufacturer specified performance targets include: lightweight, minimal use of supplied oxygen, easily transportable.
Vetted Performance Status
Unknown
Safety
The system should be operated by a trained medical professional that can assess the needs of the patient and adjust the system accordingly.
Complementary Technical Systems
The system is battery operated and as such requires a power source to be recharged. The system can take an input between 100-240V. The system can be used in conjunction with Diamedica's anesthesia machines.
Academic Research and References
C. E. Cox, S. S. Carson, J. H. Lindquist, M. K. Olsen, J. A. Govert, and L. Chelluri, Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: A prospective cohort study, Crit. Care, vol. 11, no. 1, pp. 1–11, Jan. 2007, doi: 10.1186/cc5667.
M. Unroe et al., One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: A cohort study, Ann. Intern. Med., vol. 153, no. 3, pp. 167–175, Aug. 2010, doi: 10.7326/0003-4819-153-3-201008030-00007.
M. A. Rojas et al., Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: A randomized, controlled trial, Pediatrics, vol. 123, no. 1, pp. 137–142, Jan. 2009, doi: 10.1542/peds.2007-3501.
Compliance with regulations
CE certified
Evaluation methods
Unknown
Other Information
Won the International Innovation of the Year and the Solution of the Year awards at the 2017 Africa Healthcare Summit.

Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
February 2, 2024
Implemented by
Gradian Health Systems
Agriculture
March 8, 2024
Implemented by
Laerdal Global Health

Agriculture
March 8, 2024
Implemented by
MTTS

Agriculture
March 8, 2024
Implemented by
Mechanical Ventilator Milano

Agriculture
March 8, 2024
Implemented by
Conseil Européen pour la Recherche Nucléaire (CERN)

Agriculture
February 16, 2024
Implemented by
Eniware

Agriculture
November 30, 2024
Implemented by
Sustainable Community Development Services (SCODE)

Agriculture
January 30, 2024
Implemented by
Puralytics
Agriculture
March 8, 2024
Have thoughts on how we can improve?
Give Us Feedback