Agriculture
February 21, 2024
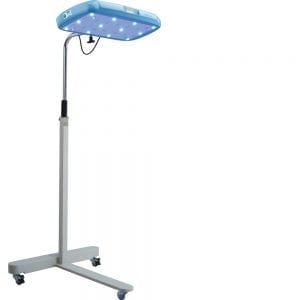
Brilliance Phototherapy Device
Read SolutionImplemented by
Equalize Health
Updated on February 8, 2024
·Created on June 4, 2014
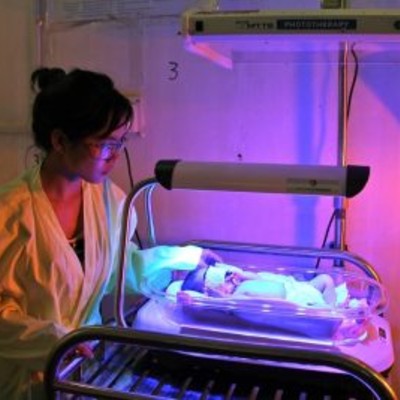
This device is for treating neonates with jaundice caused by a high level of bilirubin.
Firefly is an intuitive phototherapy device designed to treat newborns with jaundice in low-resource settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$1,500.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
East Meets West Foundation through its Breath of Life program, MTTS for direct sales.
Competitive Landscape
Direct competitors include Little Sparrows Technologies Bili-Hut, D-Rev Brilliance Pro, and Brilliance Phototherapy Device.
Regions
Africa, Asia, Caribbean
Countries
Afghanistan, Benin, Burkina Faso, Burundi, Cambodia, Ghana, Indonesia, Laos, Malaysia, Mongolia, Nepal, Nigeria, Philippines, Sierra Leone, Singapore, Somalia, South Africa, Tanzania, Thailand, Timor-Leste, Uganda, Vietnam, Zimbabwe
Manufacturing/Building Method
Firefly is in full-scale manufacturing. All components of the Firefly have been designed to be mass-produced. Design that Matters has provided capacity development for Vietnamese manufacturer Medical Technology Transfer Services (MTTS) to assist with the sourcing of mass-produced plastic parts and in-house metal tube bending capabilities so that MTTS can mass-produce the Firefly Interview with designer
Intellectural Property Type
Trade Secret
User Provision Model
DtM and partners MTTS and Day One Health continue to work with individual donors, corporate sponsors, international aid groups and government agencies to distribute Firefly to hospitals and new countries and conduct user training. To date the devices have been supplied to Asia, Africa and the Caribbean region.
Distributions to Date Status
As of November 2018, the Firefly phototherapy device has treated more than 200,000 newborns in 25 countries across Africa, Asia, and the Caribbean including; Vietnam, Myanmar, Cambodia, East Timor, Thailand, Malaysia, Ghana, Jamaica, Uganda, Zimbabwe, and Tanzania. East Meets West, MTTS, and DtM continue to expand Firefly into new hospitals and countries, and seek out new distribution channels.
Average spectral irradiance (μW/cm2/nm)
23 (Top Light) 34 (Bottom Light)
Device dimensions
66 cm x 38 cm x 49.5 cm
Device weight (kg)
11.8 kg
Effective surface area (cm)
24 cm x 53 cm (Top Light) 40 cm x 48 cm (Bottom Light)
Illumination source
Blue LEDs (High-power, 1-1.25 W)
Irradiance uniformity ratio
0.51 (Top Light (IEC Compliant >0.4)) 0.72 ( Bottom Light (IEC Compliant >0.4))
Lamp duration (hrs)
44,000 hrs (running 24/7)
Max power consumption (W)
240V, 30W
Peak wavelength (nm)
455 – 465 nm
Recommended treatment distance (cm)
Fixed distance (unknown)
Time totalizer (machine run/therapy)
Machine run, therapy time
Voltage type
24 VDC (includes off-the-shelf external power supply)
Design Specifications
Component Overview
Firefly consists of a removable bassinet made from clear scratch-resistant plastic, a fixed top light, a bottom light, and a control panel for displaying power switch, light intensity, treatment hours, and total device hours.
Key Features: No moving parts; enclosure designed toward NEMA Type 5
Dimensions (LxWxH): Overall (66 cm x 38 cm x 49.5 cm ), Top Light ( 51.6 cm x 12.5 cm x 6.3 cm), Bottom Light (64.7 cm x 38 cm x 10 cm)
Total unit mass: 11.8 kgs
Operational Overview: 2-sided light design offers top and bottom phototherapy reducing total treatment time; the portable and compact nature of Firefly allows it to be used in mother's recovery room so she can continue breastfeeding while infant receives phototherapy; single power button with single light intensity setting and fixed light distance is intended to keep the Firefly simple and to minimize the risk of using it incorrectly.
Technical Support
Medical staff must be trained in proper use. MTTS provides user training through a network of consultants located in the different countries
Replacement Components
Firefly was designed with minimalism in mind with few components that could require replacement like fuses, power cables, AC plug, and Trip switch. The LED lights used are expected to last for five years with non-stop usage 24/7
Lifecycle
The Firefly has been designed for 5 years of 24/7 hour operation. Its LED lights are rated to last up to 44,000 hours before requiring replacement. In addition, Firefly has no moving parts, outer casings have tight seams and no holes in order to prevent most insects, dust and liquids from entering and damaging the device or dimming the lights.
MTTS offers a 1 year warranty for all of the products it manufactures
Manufacturer Specified Performance Parameters
FireFly set out to develop an easy-to-use, durable, portable, and compact phototherapy device for use in resource-limited settings.
Vetted Performance Status
Firefly data from 17 infant treatments with average initial TSB 305 uMol/L (17.8 mg/dL) yielded median serum bilirubin reduction rate of -6.7 uMol/L/hr (-0.39 mg/dL/hr). Multiple linear regression of Firefly data based on study of 66 infants in 2-sided phototherapy predicts Firefly rate of -5.3 uMol/L/hr (-0.31 mg/dL/hr) given initial TSB of 265 uMol/L (15.5 mg/dL) A Clinical Evaluation of 17 infant treatments indicated Firefly reduces treatment time for a typical patient by at least 40% compared to state-of-the-art single-sided LED phototherapy. Reduced treatment time meant discharge from the highly infectious hospital environment after 20 hours on average instead of the typical 35 hours or often much longer in low-resource settings. Clinical trials continued: As of July 18, 2012, local clinical trial doctors reported 3 out of 55 infants treated with Firefly would have had to undergo an exchange blood transfusion if Firefly had not been available. A recent peer-reviewed study (Arnolda et al., PLOS One, 2018) summarizes the results of a two-year side-by-side comparison of phototherapy performance between single- and double-sided devices conducted at Danang Women and Children's Hospital in Vietnam. Compared to the GE Lullaby overhead phototherapy device, Firefly showed a 54% increase in the hourly rate of bilirubin (TSB) reduction and an overall 21% decrease in newborn treatment time.
Safety
Firefly cannot be used with overhead infant warmers due to the design (it can be used with side warmers). This means that it is better suited for use in warm climates or for treating term infants who have jaundice but can regulate their own body temperature.
Complementary Technical Systems
Eye covers; Gonad covers
Academic Research and References
Maisels, M.J., et al., 2007, “Randomized controlled trial of light-emitting diode phototherapy,” Journal of Perinatology, 27(9), pp. 565-7.
Johansen E., 2018, “Making Human Factors Affordable for Medical Device and Global Health Startups,” Proceedings of the International Symposium on Human Factors and Ergonomics in Health Care. 7(1), pp. 140–147.
Taylor, A., et al., 2016, “Innovating for global health: Study of healthcare technology failure in southern Malawi,” Annals of Global Health, 82(3), pp. 600.
Arnolda, G., et al., 2018, “A comparison of the effectiveness of three LED phototherapy machines, single-and double-sided, for treating neonatal jaundice in a low-resource setting,” PloS one, pp. 13(10), e0205432.
Onwe, O. E., Ezeanosike, O. B., and Obu, C. D., 2019, “Economic cost savings with the use of total body phototherapy for the treatment of severe neonatal jaundice in Nigeria,” J Pediatr Neonatal Care, 9(6), pp. 168-171.
Johansen, E., 2018, “Making Human Factors Affordable for Medical Device and Global Health Startups,” In Proceedings of the International Symposium on Human Factors and Ergonomics in Health Care, Vol. 7, No. 1, pp. 140-147.
Fisher, M. J., and Johansen, E., 2020, “Human-centered design for medical devices and diagnostics in global health,” Global Health Innovation, 3(1).
MMTS Lifekit, “Firefly phototherapy”
Design that matters, “Firefly Design overview”
Design that matters, “Firefly Case Study”
Technology exchange lab, “Firefly Newborn Phototherapy Device“
Compliance with regulations
IEC 60601-1:2005: Medical Electrical Equipment - Part 1: General Requirements
for Basic Safety and Essential Performance
IEC 60601-2-50:2009: Medical Electrical Equipment - Part 2-50: Particular requirements
for the safety of infant phototherapy equipment
American Academy of Pediatrics phototherapy standard: >30 uW/cm2/nm
Evaluation methods
Rate of breakdown in Serum Bilirubin levels
Reduction in treatment and Discharge time for children with Jaundice
Other Information
None

Agriculture
February 21, 2024
Implemented by
Equalize Health

Agriculture
January 13, 2024
Implemented by
Barefoot Power

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 10, 2024
Implemented by
Disease Diagnostic Group

Agriculture
January 3, 2024
Implemented by
Stanford Researchers and the International Centre for Diarrhoeal Disease Research, Bangladesh

Agriculture
February 5, 2024
Implemented by
Circ MedTech
Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360
Agriculture
December 12, 2023
Implemented by
Sohum Innovation lab
Agriculture
December 19, 2023
Implemented by
Star Syringe

Agriculture
September 26, 2024
Implemented by
Jorge Odón
Have thoughts on how we can improve?
Give Us Feedback