
Agriculture
February 29, 2024
ShangRing Circumcision Device
Read SolutionImplemented by
Jian Zhong Shang

Updated on February 5, 2024
·Created on August 27, 2015
Prepex is a tool for performing non-surgical circumcisions.
The Prepex Circumcision Device is a medical device which facilitates non-surgical adult male circumcision without the use of anesthesia, surgery, suturing or sterile setting. It is designed to improve and advance the circumcision experience for the patient and the related ecosystem, in order to achieve rapid scale-up of voluntary medical male circumcision (VMMC) in resource limited settings and reduce transmission of HIV/AIDS and other infectious diseases.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$20.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Circ MedTech Ltd distributes the Prepex device to health clinics and hospitals.
Competitive Landscape
Direct competitors include ShangRing Circumcision Device.
Regions
Africa, Sub-Saharan Africa
Countries
Botswana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Rwanda, South Africa, Swaziland, Tanzania, Uganda, Zambia, Zimbabwe
Manufacturing/Building Method
The Prepex device is mass produced by Circ MedTech, Ltd.
Intellectural Property Type
Patent
User Provision Model
This device is available in health clinics and hospitals for use by surgically trained nurses and/or minimally skilled healthcare workers.
Distributions to Date Status
In 2011, Prepex and the Rwandan Military hospital successfully tested the device and 10,000 procedures were performed.
Age Requirement
18+
Infection Rate (%)
Unknown.
Injection of local anesthesia required (Y/N)
No
Number of visits per procedure
2
Procedure Time (“skin to skin”)
2.5-3 minutes (Placement and Removal)
Design Specifications
The Prepex Circumcision Device consists of an inner ring, elastic ring, placement ring, and a verification thread; there is also a sizing accessory, a template with 5 holes of differing size to guide the selection of ring size. The device works by compressing the foreskin so as to cut off circulation distally, after which the distal foreskin becomes necrotic, allowing easy and bloodless removal.
Technical Support
Healthcare professionals are trained over a period of 3 days to 2 weeks. A Prepex training program is available to physicians.
Replacement Components
None
Lifecycle
The components are created for single use and disposal.
Manufacturer Specified Performance Parameters
The PrePex device enables non-surgical adult male circumcision.
Vetted Performance Status
Mean time to complete healing was 25.3 days after foreskin removal or 32 days after device placement. The PrePex device was safe and effective as a means of performing bloodless adult male circumcision that can be carried out by nonphysician staff without need for anesthesia, suturing, or sterile settings. The mean PrePex skin-to-skin procedure time is 3.1 minutes. 91 of 92 patients (99%) in the PrePex group were satisfied with the aesthetics of circumcision. Ninety-one of 92 (99%) patients in the PrePex group would recommend the procedure.
Safety
This device is made for single use and disposal. The PrePex device should not be used in cases of phimosis or congenital abnormality.
Complementary Technical Systems
None
Academic Research and References
Mutabazi V, Kaplan S, Rwamasirabo E, Bitega JP, Ngeruka ML. Male Circumcision Comparison Between a Nonsurgical Device to a Surgical Technique. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012;61(1):49–55.
Duffy K, Galukande M, Wooding N, Dea M, Coutinho A. Reach and Cost-Effectiveness of the PrePex Device for Safe Male Circumcision in Uganda. PLoS ONE. 2013;8(5). doi:10.1371/journal.pone.0063134
Galukande M, Duffy K, Bitega JP, Rackara S, Bbaale DS, Nakaggwa F, Nagaddya T, Wooding N, Dea M, Coutinho A. Adverse Events Profile of PrePex a Non-Surgical Device for Adult Male Circumcision in a Ugandan Urban Setting. PLoS ONE. 2014;9(1). doi:10.1371/journal.pone.0086631
Editor, Circlist. “The PREPEX Device.” CIRCLIST, 2015.
Compliance with regulations
PrePex is WHO prequalified, FDA cleared and certified CE Mark Class IIa. Circ MedTech is ISO 13485 certified.
Evaluation methods
Randomized, Controlled Intervention Trials, Government-sponsored studies. User feedback, healing time, skin-to-skin procedure time.
Other Information
As of February 2014 PrePex is being used exclusively in 13 priority countries in Sub-Saharan Africa with high HIV prevalence and consequently the device is not yet available elsewhere.

Agriculture
February 29, 2024
Implemented by
Jian Zhong Shang

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 10, 2024
Implemented by
Disease Diagnostic Group

Agriculture
January 3, 2024
Implemented by
Stanford Researchers and the International Centre for Diarrhoeal Disease Research, Bangladesh
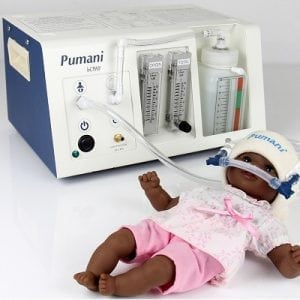
Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360

Agriculture
November 22, 2023
Implemented by
The NeoRest team at the Seattle Children’s Hopsital
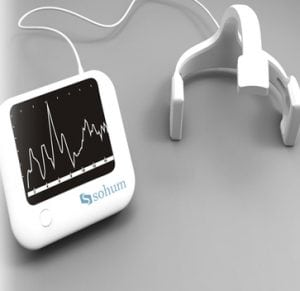
Agriculture
December 12, 2023
Implemented by
Sohum Innovation lab
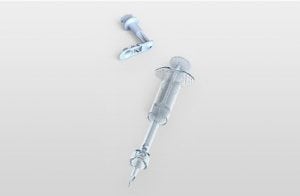
Agriculture
December 19, 2023
Implemented by
Star Syringe

Agriculture
September 26, 2024
Implemented by
Jorge Odón

Agriculture
February 26, 2024
Implemented by
Hemex Health
Have thoughts on how we can improve?
Give Us Feedback