
Agriculture
November 30, 2024
AGNI Star Rice Husk Gas Stove
Read SolutionImplemented by
NDMI Renewable Energy Private Limited
Updated on December 19, 2023
·Created on August 27, 2015
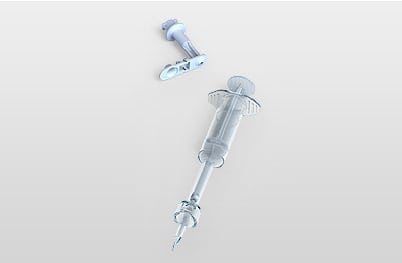
The Star ID device enables consistent accurate Intra Dermal (ID) injection while at the same time facilitating access to all vial sizes and ampules without any additional devices or manipulation.
The Star ID device enables consistent accurate Intra Dermal (ID) injection while at the same time facilitating access to all vial sizes and ampules without any additional devices or manipulation.
This product is discontinued.
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
Unknown
Regions
Worldwide
Manufacturing/Building Method
Mass produced via a licensee network of ISO quality, clean room and production facilities located all over the world.
Intellectural Property Type
Patent
Compliance with regulations
Meets WHO global public health standards for auto-disable technology and US standards for needle stick protection

Agriculture
November 30, 2024
Implemented by
NDMI Renewable Energy Private Limited

Agriculture
June 23, 2024
Implemented by
Star 8 Green
Agriculture
February 21, 2024
Implemented by
Equalize Health
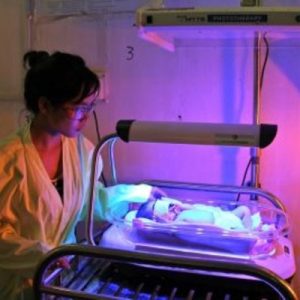
Agriculture
February 8, 2024
Implemented by
Design That Matters (DtM)

Agriculture
January 3, 2024
Implemented by
Stanford Researchers and the International Centre for Diarrhoeal Disease Research, Bangladesh
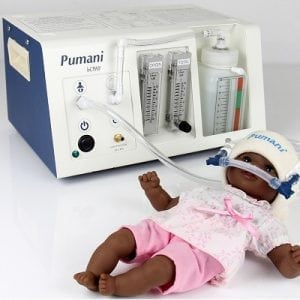
Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360

Agriculture
November 22, 2023
Implemented by
The NeoRest team at the Seattle Children’s Hopsital
Agriculture
December 12, 2023
Implemented by
Sohum Innovation lab

Agriculture
September 26, 2024
Implemented by
Jorge Odón

Agriculture
February 26, 2024
Implemented by
Hemex Health
Have thoughts on how we can improve?
Give Us Feedback