
Agriculture
December 7, 2023
Solar Ear Hearing Aid HD-48
Read SolutionImplemented by
Solar Ear
Updated on December 12, 2023
·Created on September 7, 2016
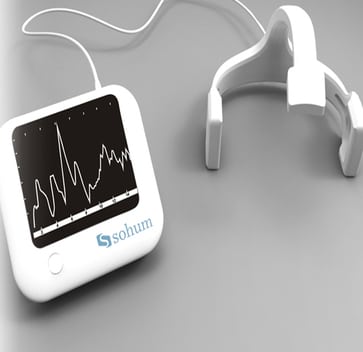
The Sohum hearing screening device is a non-invasive device that uses brainstem auditory evoked response (BAER or ABR) technology to screen neonates for hearing impairment.
The Sohum hearing screening device is a non-invasive device that uses brainstem auditory evoked response (BAER or ABR) technology to screen neonates for hearing impairment. It can screen both ears simultaneously, withstands background noise, does not require sedation, and is user-friendly for resource-constrained settings.
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Community, Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Sohum Innovation Lab, All India Institute Of Medical Science (AIIMS), St.John's Medical College Hospital, Childrens Hospital in Hyderabad, Union Ministry of Science and Technology
Competitive Landscape
Direct competitors include SHOEBOX Audiometry.

Agriculture
December 7, 2023
Implemented by
Solar Ear
Agriculture
December 7, 2023
Implemented by
ALIMCO
Agriculture
December 11, 2023
Implemented by
Solar Ear

Agriculture
February 22, 2024
Implemented by
Solar Ear
Agriculture
February 21, 2024
Implemented by
Equalize Health

Agriculture
January 3, 2024
Implemented by
Stanford Researchers and the International Centre for Diarrhoeal Disease Research, Bangladesh
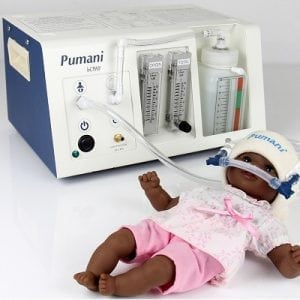
Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360
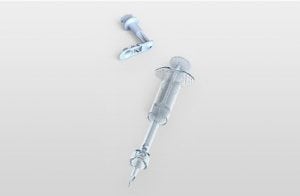
Agriculture
December 19, 2023
Implemented by
Star Syringe

Agriculture
September 26, 2024
Implemented by
Jorge Odón

Agriculture
February 26, 2024
Implemented by
Hemex Health
Have thoughts on how we can improve?
Give Us Feedback