
Agriculture
February 14, 2024
DFA Paper-based liver function test (AIDS, TB)
Read SolutionImplemented by
Diagnostics for all
Updated on February 26, 2024
·Created on September 1, 2021
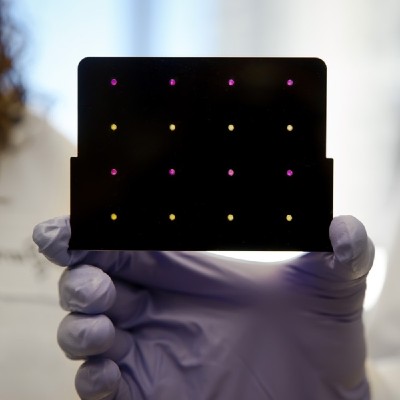
A paper-based diagnostic device to detect viral threats
A Zika paper-based diagnostic device is a molecular diagnostic tool embedded into paper that uses blood, urine or saliva to provide results in a short time. It is composed of three components: An amplifier for the genetic sequences found in the patient sample; a toehold switch sensor to recognize if the sequences found are from Zika virus, and a third component to determine the strain of the virus. This product is still a proof of concept, and additional testing is needed to ensure safety and efficacy before actual deployment.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$1.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Unknown
Regions
Caribbean, Central America, South America
Manufacturing/Building Method
This device takes approximately 5 days to be manufactured at the synthetic biology laboratories from the Wyss Institute for Biologically Inspired Engineering.
Intellectural Property Type
Patent
User Provision Model
Product not commercially available yet.
Distributions to Date Status
None
Consumables
Paper tests
Detection sensitivity
98% sensitivity -similar to Zika CDC RT-qPCR-, and 100% accuracy to differentiate Zika from Dengue.
Indispensable equipment for function (Y/N)
Electronic optical reader
Maintenance or calibration required by user at time of use? (Y/N)
No
Number of Tests Performed
1
Power supply type: Continuous, Recharging only (V, time required, battery life), Other
Not required
Time required for procedure (minutes)
3 hours
Design Specifications
Following the World Health Organization (WHO) criteria for rapid diagnostic tests, researchers aimed to create a sterile and abiotic platform that can be utilized outside of laboratory conditions without concern over biosafety.
Requirements:
Technical Support
Provided by manufacturer.
Replacement Components
N/A
Lifecycle
Single use, disposable test. 1 year in storage.
Manufacturer Specified Performance Parameters
Testing time is less than 3 hours. The amplification and DNA detection process uses a NASBA (nucleic acid sequence based amplification) due to its high sensitivity. The chance for false-positive results due to contamination are minimized by the use of sequence-specific toehold switch sensors and CRISPR/ Cas9-mediated selection downstream of the amplification. This paper-based test is able to detect 2.8 fM Zika concentrations in plasma samples, which make it clinically relevant. It is fast, easy-to-use, and requires no costly qPCR machine and PCR infrastructure, no cold-chain required. Only needs an electronic optical reader, which was also designed for low resource settings.
Vetted Performance Status
Tests were performed by the manufacturer, collecting samples from three Latin American countries: Ecuador, Brazil and Colombia.The device showed high specificity against similar regional viruses (Dengue and Chikungunya), similar sensitivity when compared to RT-qPCR for the Zika detection, and a diagnostic accuracy of 98.5% with 268 patient samples.
Safety
Additional testing would be needed to ensure safety and efficacity before actual deployment of this device.
Complementary Technical Systems
Electronic optical reader
Academic Research and References
Pardee K., 2016, “Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components,” Cell, 165 (5), pp. 1255-1266
Hall R., Macdonald J., 2016, “Synthetic Biology Provides a Toehold in the Fight against Zika,” Cell Host & Microbe, 19(6), pp. 752-754,
Silva SJRD., Pardee K., Pena L., 2019, “Loop-Mediated Isothermal Amplification (LAMP) for the Diagnosis of Zika Virus: A Review,” Viruses,12(1):19.
da Silva, S.J.R., Pardee, K., Balasuriya, U.B.R. et al., 2021, “Development and validation of a one-step reverse transcription loop-mediated isothermal amplification (RT-LAMP) for rapid detection of ZIKV in patient samples from Brazil,” Sci Rep, 11, pp. 4111
Karlikow, M., Silva, S. J. R. D., Guo, Y., Pardee, K., 2021, Developing a lab-in-a-box and low-cost paper-based sensors for ZIKV and CHIKV diagnosis in Latin America. V International Symposium of Immunologicals.
Silva, S. J. R. D., Mendes, R. P. G., Pena, L., 2021, Low-cost protocol for rapid detection of ZIKV from patient and mosquito samples using a direct-RT-qPCR assay without RNA extraction step. V International Symposium of Immunologicals.
Karlikow, Margot, “Developing a lab-in-a-box and low-cost paper-based sensors for ZIKV and CHIKV diagnosis in Latin America“, V International Symposium, 2021
Compliance with regulations
Unknown
Evaluation methods
Field trial of the paper-based system for Zika diagnostics in endemic countries. The test matched the Zika CDC RT-qPCR in specificity and sensitivity, with a comparable accuracy of 98%.
Other Information
Similar applications of this diagnostic platform have been made for Chikungunya and COVID-19 by the same researcher.

Agriculture
February 14, 2024
Implemented by
Diagnostics for all

Agriculture
March 10, 2024
Implemented by
Disease Diagnostic Group
Agriculture
February 14, 2024
Implemented by
USTAR Biotechnologies (Hangzhou) LTD
Agriculture
March 11, 2024
Implemented by
OrthoTrauma

Agriculture
February 26, 2024
Implemented by
Hemex Health

Agriculture
January 4, 2024
Implemented by
Promethean

Agriculture
March 8, 2024

Agriculture
January 22, 2024
Implemented by
Sanivation

Agriculture
February 21, 2024
Implemented by
Global Alliance for Disaster Risk Reduction & Resilience in the Education Sector

Agriculture
January 17, 2024
Implemented by
mWater
Have thoughts on how we can improve?
Give Us Feedback