
Agriculture
June 8, 2024
Mobile Obstetrics Monitoring (MOM)
Read SolutionImplemented by
Philips
Updated on March 8, 2024
·Created on August 31, 2021
Monitors and detects changes in pulmonary gas exchange in diseased states. Courtesy of WHO Compendium 2021
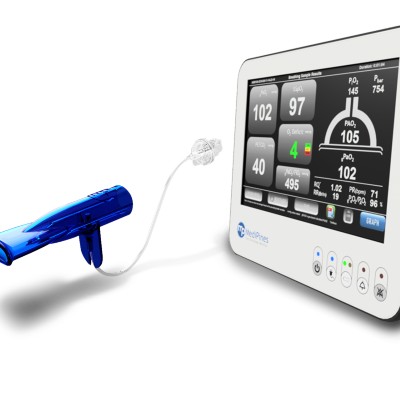
The MediPines AGM100 provides real-time measurements of 12 respiratory metrics by sampling a patient’s normal breathing and can be used to detect respiratory impairments such as those resulting from COVID-19. The device is non-invasive, portable, and displays all respiratory measurements in one output screen.
This product was selected for inclusion in WHO’s 2021 Compendium of Innovative Health Technologies for Low‐Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
This product is available from the manufacturer.
Competitive Landscape
Direct competitors include Lifebox Pulse Oximeter.
Regions
Worldwide
Manufacturing/Building Method
Manufactured in the United States
Intellectural Property Type
Patent
User Provision Model
Users can obtain the product directly from the manufacturer.
Distributions to Date Status
As of October 2020, between 0-100 units have been distributed.
Consumables
MediPines Single Patient Use Breathing Circuit Kit (AGM316), noseclip
Power supply type
Battery or continuous power supply
Indispensable equipment for function
SpO2 sensor, MediPines Single Patient Use Breathing Circuit Kit, Noseclip
Maintenance or calibration required by user at time of use? (Y/N)
No
Display Type
LCD
Gas Flow Range (L/min)
N/A
Pressure Range (cm H2O)
N/A
Patient population (specify)
Adults
Design Specifications
While wearing a pulse oximeter and nose clip, the patient breathes normally through a provided breathing circuit. Over the course of the 2-minute test, the monitor will display either real-time breathing gas waveforms or clinical parameters. After the test, the results can be saved or printed. The device is portable and can utilize its internal rechargeable battery when not connected to a direct power source. The backup battery can provide up to 2 hours of power at full charge, and takes 4 hours to fully recharge the battery. The device weighs ~ 1.7 kg.
Technical Support
Provided by the manufacturer.
Replacement Components
The oxygen sensor is available on the open market and can also be purchased through MediPines.
Lifecycle
0-2 year lifetime, includes a 1-year warranty
Manufacturer Specified Performance Parameters
The manufacturer specified performance targets include automatic calibration, high accuracy, 1-2 minute reading turnaround, non-invasive, push-button operation, portable, economical, and efficient.
Vetted Performance Status
Testing performed by academic institutions found that the device "provided a valid and reliable measure against directly measured arterial blood gasses at rest and during hypoxic exercise". Another study found that this device is "very sensitive to the presence of disease and may discard the need for arterial punctures in many instances" for patients with lung disease. Additionally, this device has been tested for water ingress/mechanical strength testing, Electrical Safety testing (60601-1-2), and Medical Device Safety Standards Testing (60601-1, 80601-2-55, 80601-2-61).
Safety
The device should be operated by a trained individual with appropriate decontamination between use.
Complementary Technical Systems
SpO2 sensor, MediPines Single Patient Use Breathing Circuit Kit, Noseclip, and power source for charging the battery/use.
Academic Research and References
Howe, C., et. al, 2020, Validation of a Non-invasive Assessment of Pulmonary Gas Exchange During Exercise in Hypoxia. Chest, Vol. 158(4), pp. 1644-1650.
West J.B., et. al, 2018, A New, Noninvasive Method of Measuring Impaired Pulmonary Gas Exchange in Lung Disease: An Outpatient Study. Chest. Vol. 154(2), pp. 363-369.
Compliance with regulations
AAMI / ANSI ES60601-1:2005/ (R)2012 + A1:2012 + C1:2009/(R)2012 + A2:2010/(R)2012
IEC 60601-1-2:2014
ISO 80601-2-61: 2011
ISO 80601-2-55:2011
IEC 62304: 2006
IEC 60068-2-27:2008
IEC 60068-2-64:2008
ISO 10993-1:2009/(R)2013
ISO 10993-5:2009
ISO 10993-10:2010
ISO 14971:2007
Evaluation methods
This device is evaluated for measuring 12 respiratory metrics.
Other Information
None

Agriculture
June 8, 2024
Implemented by
Philips

Agriculture
June 23, 2024
Implemented by
Planet Labs

Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
February 16, 2024
Implemented by
Eniware

Agriculture
November 30, 2024
Implemented by
Sustainable Community Development Services (SCODE)

Agriculture
June 17, 2024
Implemented by
New America

Agriculture
January 30, 2024
Implemented by
Puralytics

Agriculture
December 18, 2024
Implemented by
Tinytech Plants

Agriculture
November 7, 2018

Agriculture
January 2, 2024
Implemented by
Uzima
Have thoughts on how we can improve?
Give Us Feedback